Azide compounds are crucial for the synthesis of important organic and materials chemistry compounds like amines and triazoles. Triazoles, which can be synthesized through the ‘click’ reaction, have gained attention in the pharmaceutical and other industries. However, the electrophilic nature of azido groups makes them vulnerable to various nucleophiles, such as carbanions. This presents a significant challenge in synthesizing carbanions with azido groups.
To address this challenge, a team of researchers from Japan, led by Associate Professor Suguru Yoshida from Tokyo University of Science (TUS), has developed an innovative and efficient method for synthesizing azides.
In their recent article published in the journal Frontiers in Chemistry, Dr. Yoshida and his colleagues, Ms. Rina Namioka (TUS) and Ms. Minori Suzuki (Tokyo Medical and Dental University), detail an efficient method for preparing organomagnesium intermediates with protected azido groups.
“We conducted this research because we believe that by developing a synthesis method based on the azide group protection method we discovered, we can expand the range of easily synthesizable azide compounds,” says Dr. Yoshida.
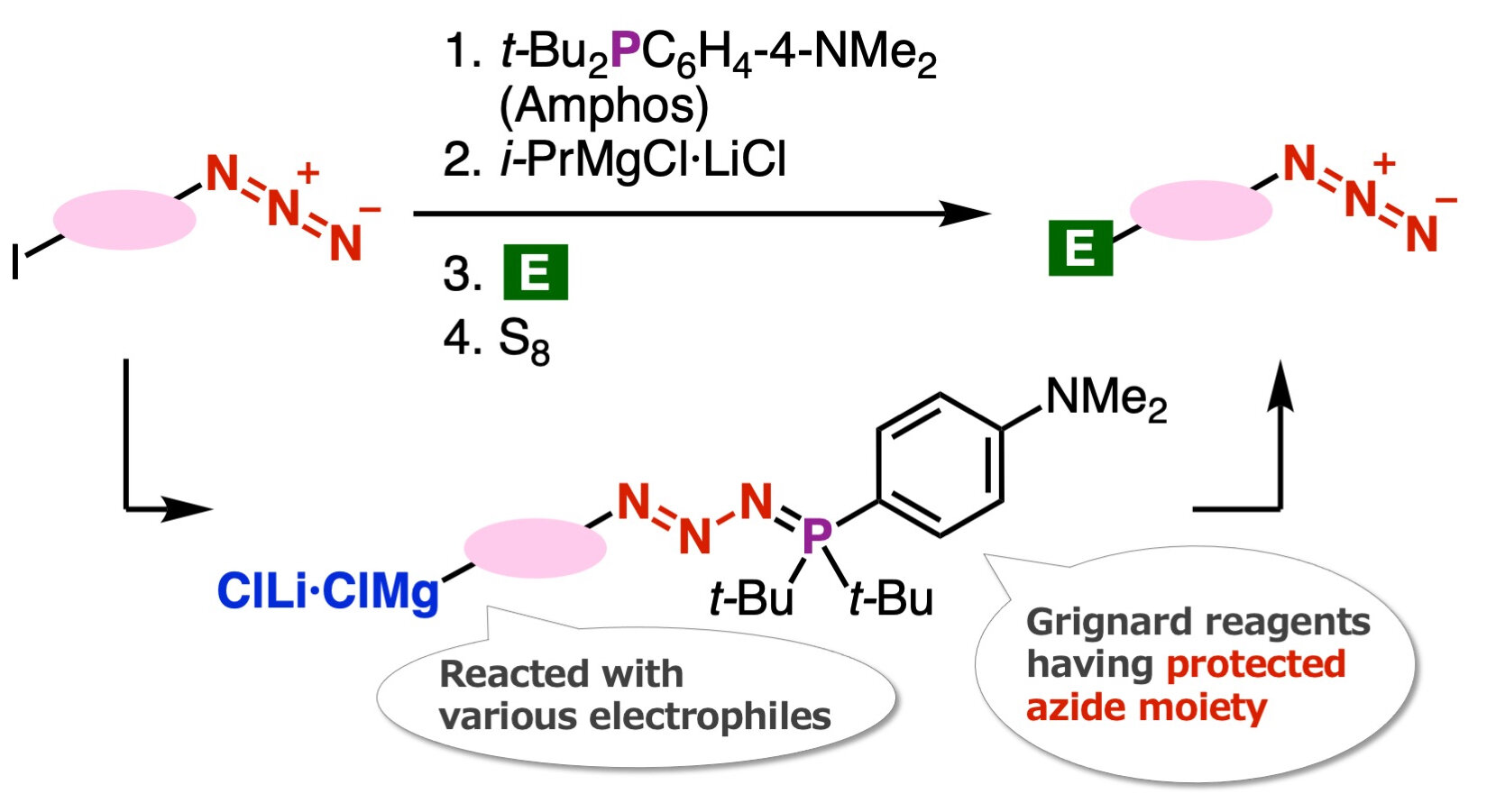
The novelty of this synthesis method lies in the protection of azido groups using di(tert-butyl)(4-(dimethylamino) phenylphosphine (Amphos) and the subsequent iodine-magnesium exchange, which enables the preparation of organomagnesium intermediates. These intermediates are then used in the synthesis of various azides through transformations with different electrophiles, followed by deprotection with elemental sulfur. The authors of this study have also developed a new method for synthesizing 1,2,3-triazoles.
The team discovered that the iodine-magnesium exchange reaction efficiently occurs with aryl iodides containing azido groups using the recently developed ‘azide group protection method’. Dr. Yoshida explains, “The Grignard reaction of the organomagnesium compounds synthesized through this reaction successfully led to the synthesis of a wide range of azide compounds.”
Since azide compounds are essential in the synthesis of 1,2,3-triazoles through click chemistry, this method is expected to contribute to the efficient development of pharmaceuticals and other industrially important products.
“Our lab is currently researching the preparation and transformation of carbanions with phosphazide moieties to expand the range of such compounds,” notes Dr. Yoshida optimistically. The seamless and efficient synthesis of azides, enabling the synthesis of a wide range of organonitrogen compounds like amines and triazoles, will greatly benefit the synthetic organic chemistry, pharmaceutical sciences, and materials chemistry communities.