Enzymes in industrial biocatalysis are revolutionizing the development of a sustainable chemical industry. These powerful catalysts can synthesize complex molecules, including pharmaceutical substances, in an environmentally friendly way.
Researchers at the Karlsruhe Institute of Technology (KIT) have made a groundbreaking discovery in the field of industrial biocatalysis. They have developed a new class of materials by creating enzyme foams that are incredibly stable and active. Their findings have been published in Advanced Materials, and they have already filed a patent application for the process.
Industrial biocatalysis is primarily used in the production of pharmaceuticals and special chemicals. To enhance this process, researchers are exploring new technologies. Enzymes, unlike traditional chemical catalysts, accelerate reactions while conserving resources and energy.
The current focus is on producing large quantities of enzyme biocatalysts under gentle conditions. To achieve efficient substance conversions, enzymes are immobilized in microstructured flow reactors. This immobilization increases enzyme concentration and productivity.
Creating Stable Enzyme Foams
Ordinarily, foaming alters the structure of enzymes and diminishes their biocatalytic activity. However, the newly developed protein foams exhibit remarkable stability and activity. Activity is a measure of how effectively enzymes facilitate the reaction between initial substances.
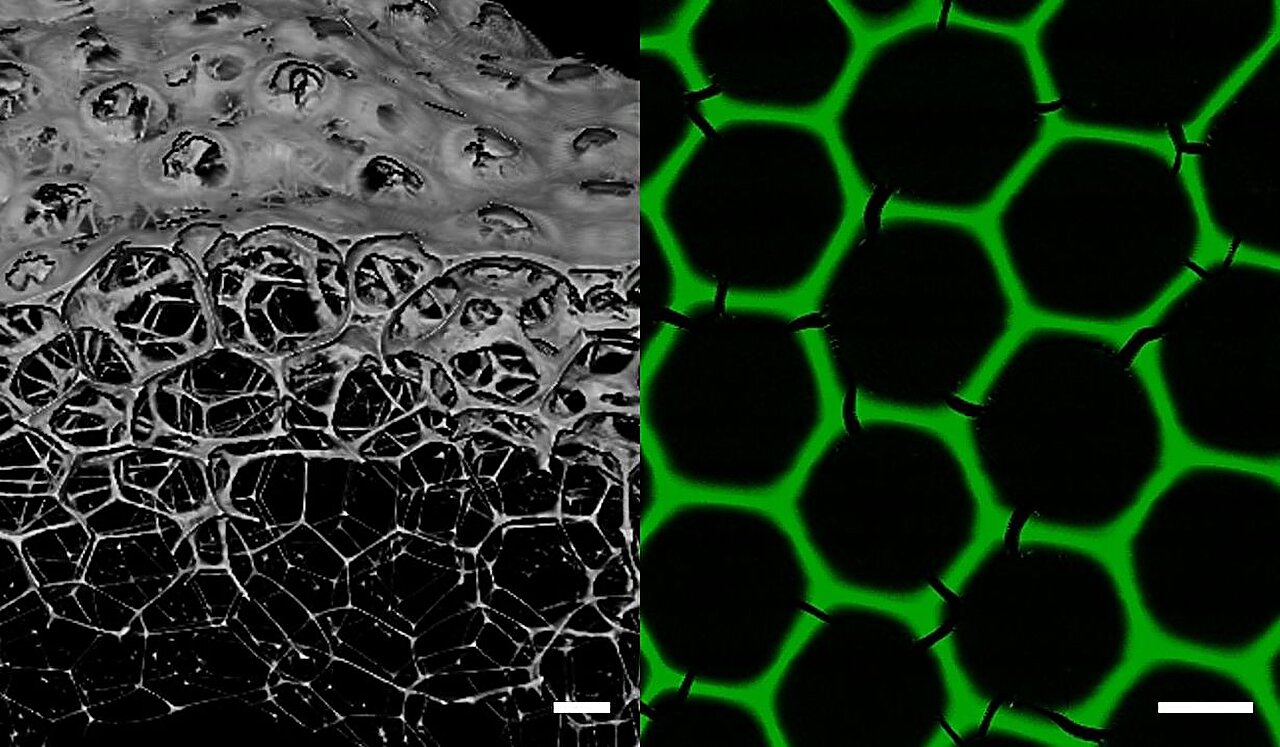
To produce protein foams, two dehydrogenase enzymes with matching sites are mixed, forming a stable protein network. A controlled gas flow is then introduced to create uniform-sized microscopic bubbles in a microfluidic chip. The foam, with its uniform bubble size, is applied to plastic chips and dried, resulting in the formation of a stable hexagonal lattice.
“These monodisperse all-enzyme foams consist exclusively of biocatalytically active proteins,” explains Professor Christof Niemeyer from the Institute for Biological Interfaces 1. The foams have a mean pore diameter of 160 µm and a lamellae thickness of 8 µm, forming a stable hexagonal honeycomb structure within minutes.
Efficient Use of Full-Enzyme Foams
To maximize the efficiency of enzyme conversion reactions, large quantities of enzymes need to be immobilized under gentle conditions while maintaining their activity. Previous methods involved immobilizing enzymes on polymers or carrier particles, which occupied valuable reactor space and potentially reduced enzyme activity. The new foam-based materials offer a larger surface area for the desired reactions to occur.
Contrary to expectations, the new foams exhibit exceptional durability, mechanical resistance, and catalytic activity. This level of stability had not been achieved in previous protein foams. The researchers attribute this stability to the matching junctions of the enzymes, which enable self-assembly and the formation of an incredibly stable material network during drying.
“Surprisingly, the newly developed enzyme foams remain far more stable after four weeks of drying compared to enzymes without foams,” says Niemeyer. “This is particularly advantageous for commercialization, as it facilitates production and delivery.”
These biomaterials open up numerous opportunities for innovation in industrial bioengineering, materials sciences, and food technology. Protein foams could be used in biotechnological processes to produce valuable compounds more efficiently and sustainably. For example, the researchers successfully used the foams to produce tagatose, a promising alternative to refined sugar.