Discovering the hidden potential of metal nanoclusters in catalytic processes is like unlocking a treasure trove of possibilities. These atomically precise cluster catalysts have the power to bridge the gap between homogeneous and heterogeneous catalysis, shedding light on the intricate relationship between structure and activity. But what about the synergistic effects between non-single active sites in these nanoclusters? It’s a question that has been largely unexplored.
In a groundbreaking study published in Nature Communications, Prof. Sun Xiaoyan and their team from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) have unraveled the mysteries of oxygen coupling between dual active sites during the oxygen evolution reaction (OER).
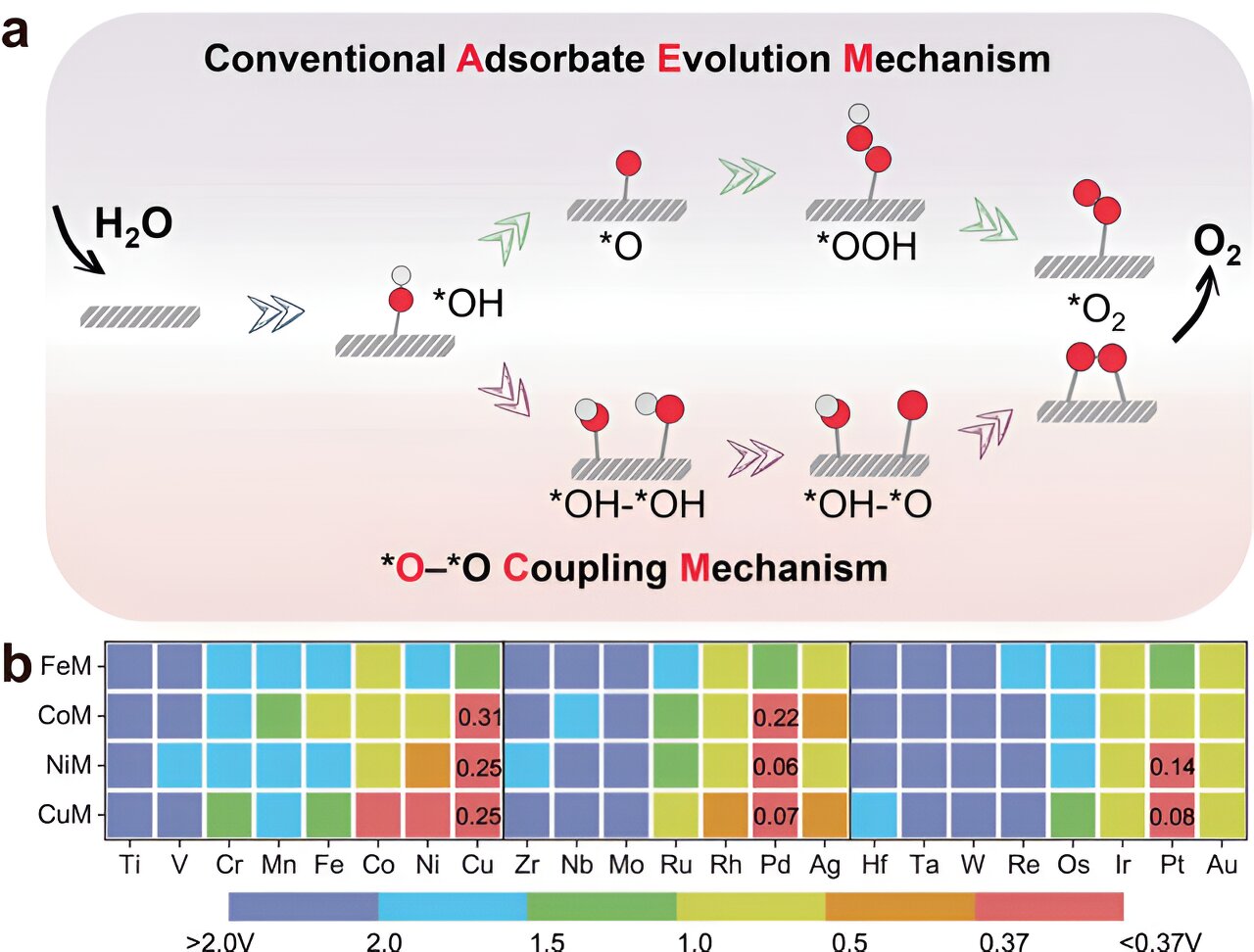
In the traditional OER mechanism, the performance is heavily reliant on the adsorption energies of oxygenated intermediates at a single active site. This limitation makes it difficult to achieve the lowest theoretical overpotential of 0.37 V. But the researchers took inspiration from the lattice oxygen mechanism and explored the potential of an *O-*O coupling mechanism (OCM) to enhance performance. Using high-throughput density functional theory (DFT) and a N-doped graphene supported dual-atom model catalyst, they were able to break through the bottleneck of 0.37 V by deviating from the conventional scaling relationship.
“These findings not only enhance our understanding of the non-single active site of cluster catalysts, but also provide valuable insights for the design of other electrochemical reaction catalysts,” explained Prof. Sun.
The research team also made significant strides in predicting high-performance homonuclear dual-atom catalysts through rationally designed activity descriptors. They also shed light on the conditions for OCM occurrence and the OER performance of the constant potential method.
“This study marks the first time that high-throughput DFT has been used to gain insight into unconventional OCM,” added Prof. Ding Yuxiao, another corresponding author. “Furthermore, our advanced computational method allows for predictions that closely resemble real electrochemical conditions.”