The precise segregation of DNA and the faithful inheritance of plasmids are crucial steps in bacterial cell division. But how exactly does this process work? A team of researchers led by Seán Murray at the Max Planck Institute for Terrestrial Microbiology has developed a groundbreaking computational simulation that sheds light on a key mechanism of DNA segregation. Their findings not only pave the way for experimental testing, but also reveal fundamental biochemical principles that have implications for synthetic biology and medical applications.
The faithful inheritance of genetic material is a fundamental process in all forms of life. At the heart of this process is the accurate transmission of copied genetic material during cell division. However, the mechanisms behind this crucial process have remained elusive. That is, until now.
Seán Murray and his team at the Max Planck Institute for Terrestrial Microbiology have successfully developed a computational simulation that provides insights into the fine structure of the proteins involved in DNA segregation. Unlike traditional experimental techniques, which have limitations in resolution, this stochastic modeling approach allows researchers to unravel the underlying processes and gain a deeper understanding of DNA segregation.
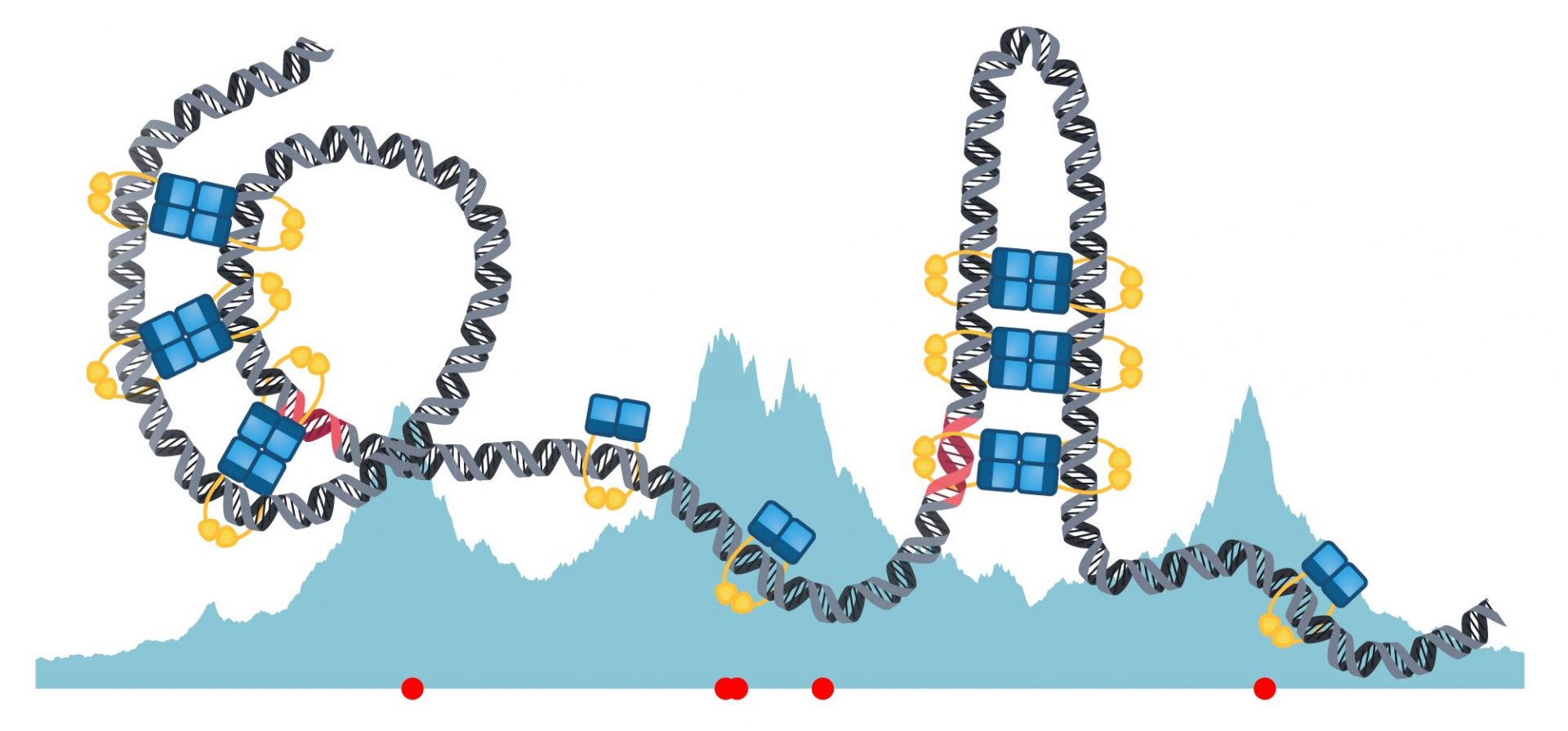
In many bacteria, a key part of this process is the formation of a large macromolecular complex called the partition complex. This complex, which is part of the ParABS system, relies on precise interactions between its protein subparts and the DNA. However, the structure and assembly mechanisms of these protein complexes have remained a mystery.
“Sliding and bridging” principle
Building on recent discoveries, Murray’s team has developed a model that reveals a fascinating “sliding and bridging” principle employed by the DNA and ParB dimers. Graduate student Lara Connolley, the first author of the study, explains that ParB dimers attach to specific regions on the DNA strand, known as parS sites, by forming a protein clamp. They then slide along the DNA, similar to beads on a chain. The model also predicts the formation of short-lived bridges that organize the DNA into hairpin and helical structures, without interfering with the sliding process.
Murray emphasizes the significance of these bridging interactions, stating that they lead to DNA bending and the formation of various structures. Further research into these structural variations could provide valuable insights into the role of ParB in different biological contexts. The study’s findings open the door for future research and experimentation to build upon these discoveries.
The next step for Murray and his team is to conduct experiments to test and validate the model predictions in more detail. Additionally, studying different bacterial species will help uncover the diversity present in the structure of the partition complex.
“Our study not only provides a deeper insight into the world of DNA segregation, but also has potential relevance for public health,” says Murray. “Low copy number plasmids, which are also segregated by the ParABS system, often carry antibiotic resistance genes. Therefore, these results could have implications beyond basic research and contribute to our understanding of antibiotic resistance.”
The findings of this groundbreaking research have been published in the prestigious journal Nature Communications.