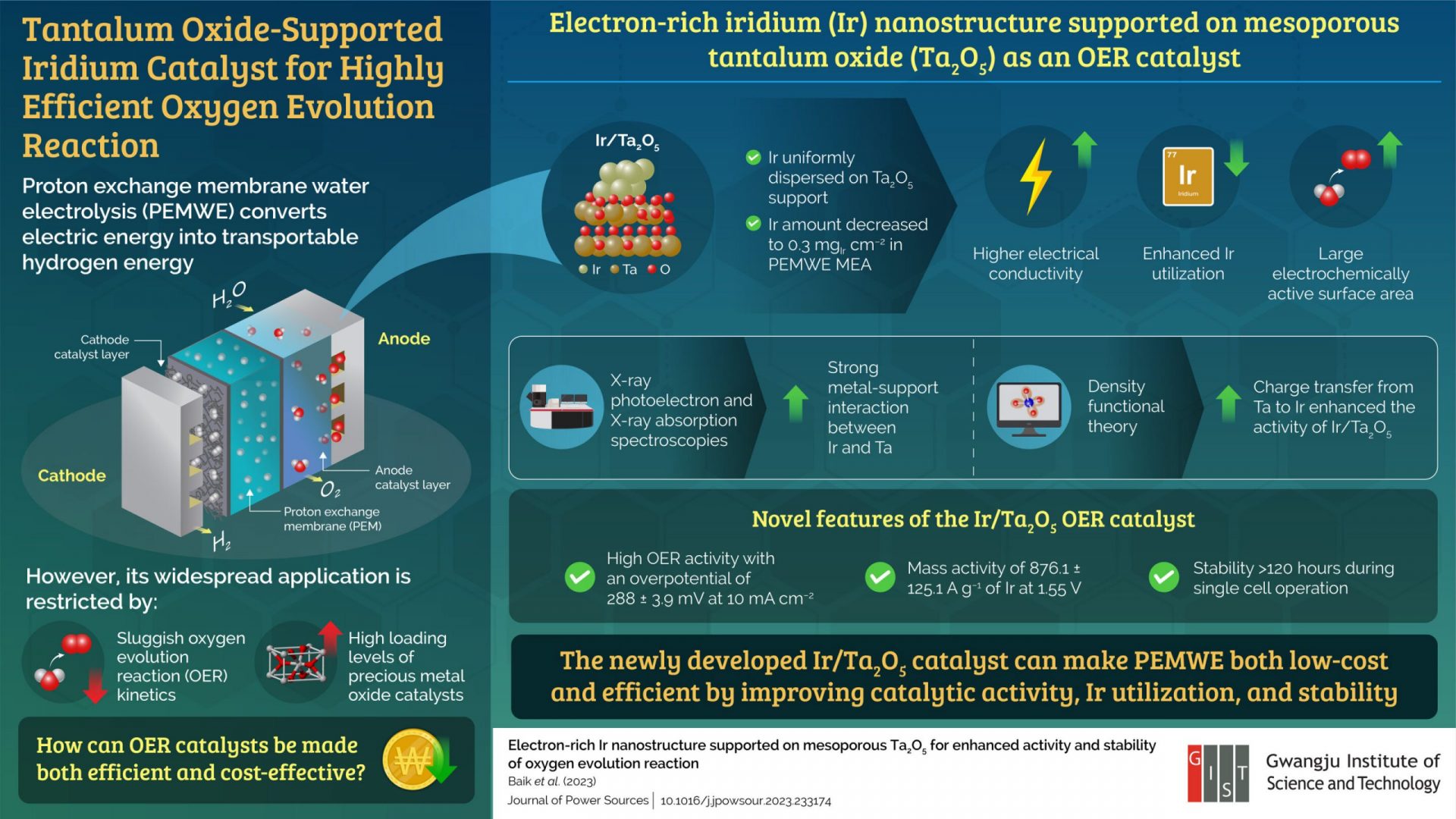
The world’s energy demands are constantly increasing, and we are in need of clean and eco-friendly solutions. One promising option is transportable hydrogen energy. Proton exchange membrane water electrolyzers (PEMWEs) have caught the attention of many as they can convert excess electric energy into transportable hydrogen energy through water electrolysis.
However, the widespread use of PEMWEs for hydrogen production is limited due to slow rates of oxygen evolution reaction (OER) and the high cost of metal oxide catalysts like iridium (Ir) and ruthenium oxides in electrodes. To overcome these challenges, it is crucial to develop cost-effective and high-performance OER catalysts.
A team of researchers from Korea and the U.S., led by Professor Chanho Pak from the Gwangju Institute of Science and Technology in Korea, has made a breakthrough in this area. They have developed a novel mesoporous tantalum oxide (Ta2O5)-supported iridium nanostructure catalyst using a modified formic acid reduction method. This catalyst has shown efficient PEM water electrolysis.
Their study, published in the Journal of Power Sources, was co-authored by Dr. Chaekyung Baik, a post-doctoral researcher at the Korea Institute of Science and Technology (KIST).
“The electron-rich Ir nanostructure was uniformly dispersed on the stable mesoporous Ta2O5 support prepared via a soft-template method combined with an ethylenediamine encircling process, which effectively decreased the amount of Ir in a single PEMWE cell to 0.3 mg cm–2,” explains Prof. Pak. This innovative Ir/Ta2O5 catalyst design not only improves the utilization of Ir but also enhances electrical conductivity and provides a larger electrochemically active surface area.
Furthermore, X-ray photoelectron and X-ray absorption spectroscopies have revealed a strong metal-support interaction between Ir and Ta. Density functional theory calculations have shown a charge transfer from Ta to Ir, resulting in a strong binding of adsorbates like O and OH. This maintains the Ir (III) ratio in the oxidative OER process and leads to the enhanced activity of Ir/Ta2O5.
The team has also demonstrated the high OER activity of the catalyst experimentally, with an overpotential of 288 ± 3.9 mV at 10 mA cm−2 and a mass activity of 876.1 ± 125.1 A g−1 of Ir at 1.55 V. These values are significantly higher than those of Ir Black. Additionally, Ir/Ta2O5 has shown excellent OER activity and stability, as confirmed through membrane electrode assembly single cell operation lasting over 120 hours.
This technology offers the dual benefit of reducing Ir loading levels and enhancing OER efficiency. “The improved OER efficiency complements the cost-effectiveness of the PEMWE process, enhancing its overall performance. This advancement has the potential to revolutionize the commercialization of PEMWEs, accelerating its adoption as a primary method for hydrogen production,” speculates an optimistic Prof. Pak.