Why an “inverse vaccine”?
Traditional vaccines aim to boost immunity, which is not ideal for treating autoimmune diseases. In these conditions, the immune system mistakenly attacks healthy cells instead of harmful ones like viruses or cancer cells.
Typically, immunosuppressants are used to treat autoimmune diseases, but they come with their own set of problems. These drugs can effectively suppress the immune system, but they also hinder the body’s ability to fight off infections, leading to numerous side effects.
Jeffrey Hubbell, the lead author of a groundbreaking study on a new vaccine, explains, “If we could treat patients with an inverse vaccine instead, it could be much more specific and lead to fewer side effects.”
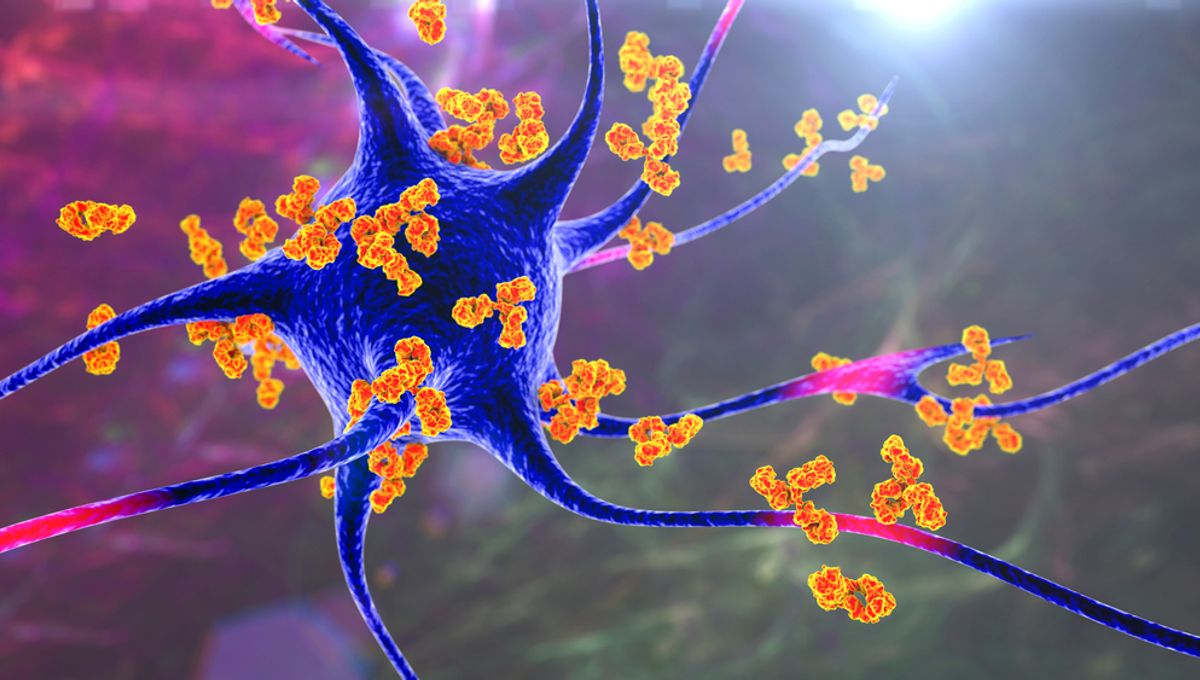
An “inverse vaccine” would essentially erase the immune system’s memory of a molecule it would normally attack. For instance, in multiple sclerosis, it would prevent immune cells from attacking myelin, the protective coating around nerve cells.
In their recent study, researchers capitalized on an existing mechanism in the body to develop such a vaccine.
The liver delivers
Under normal circumstances, the immune system doesn’t react when naturally dying cells are present. This is due to the liver’s role in tagging molecules from these cells with a “do not destroy” sign, specifically a sugar molecule called N-acetylgalactosamine (pGal).
Hubbell explains, “The idea is that we can attach any molecule we want to pGal and it will teach the immune system to tolerate it.”
Previous experiments by the research team demonstrated that this concept could prevent autoimmunity. However, their latest research suggests that it could also treat diseases that have already begun.
In a mouse model of a multiple sclerosis-like disease, the researchers used an inverse vaccine where pGal was linked to myelin proteins. The immune system ceased attacking myelin, resulting in the restoration of nerve function and the reversal of symptoms.
Hubbell expresses excitement, stating, “What is so exciting about this work is that we have shown that we can treat diseases like multiple sclerosis after there is already ongoing inflammation, which is more useful in a real-world context.”
What’s next?
To make this breakthrough a reality, further research beyond mouse models is necessary. Some phase I clinical safety trials have already been conducted using a similar method to treat celiac disease, and trials for multiple sclerosis are currently in progress.
Hubbell concludes, “There are no clinically approved inverse vaccines yet, but we’re incredibly excited about moving this technology forward.”
The study is published in Nature Biomedical Engineering.