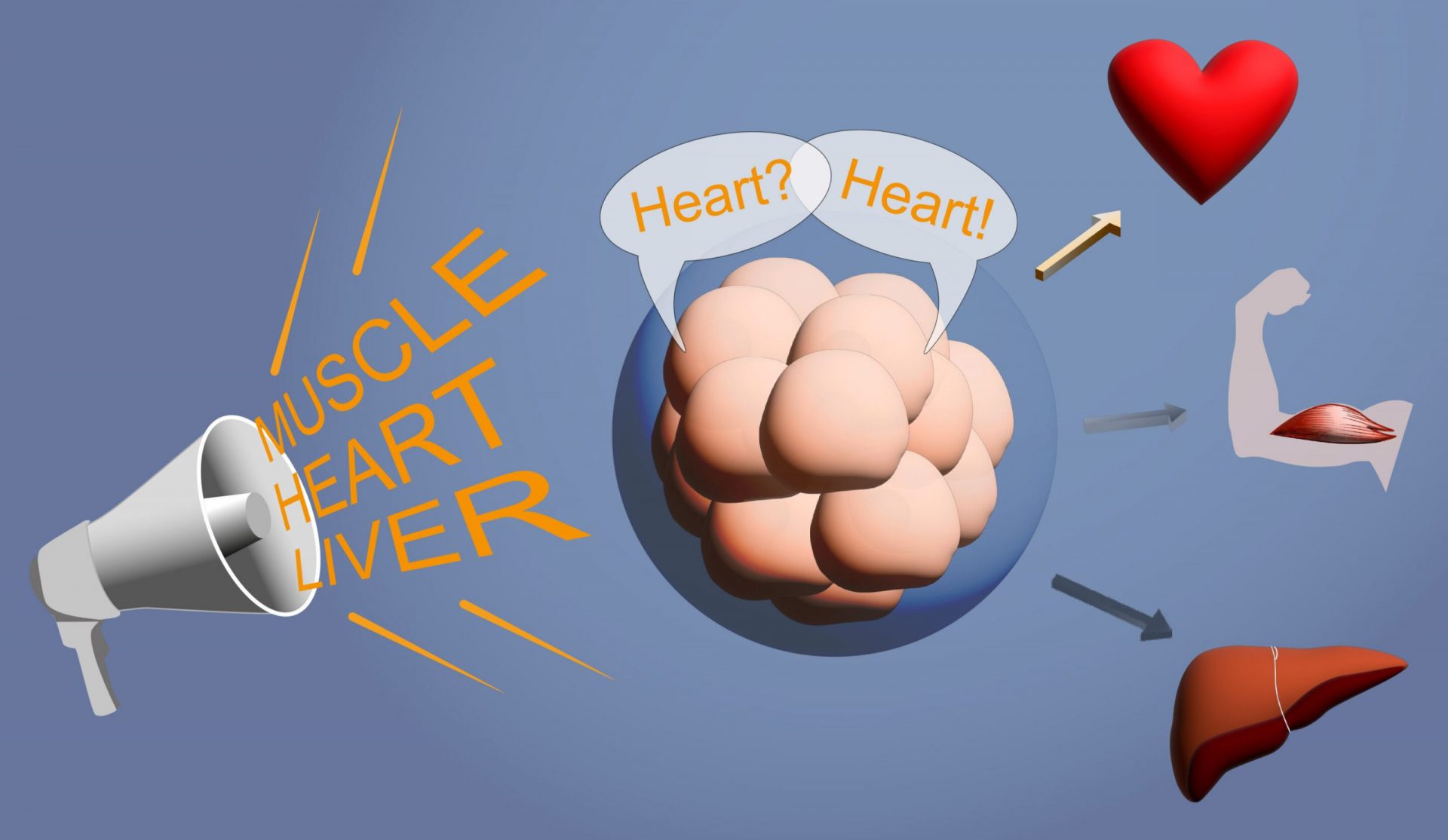
Choosing a career is like embarking on an exciting adventure, filled with endless possibilities and important decisions. Just like us, our cells also face this crucial choice during embryonic development. Some become blood cells, others muscle cells, and some even become nerve cells.
In a groundbreaking study led by Christian Schröter at the Max Planck Institute of Molecular Physiology in Dortmund, the team has uncovered the fascinating influence of two signaling molecules, FGF and BMP, on the career choices of stem cells. What’s even more intriguing is that stem cells have the power to shape their own destiny. These remarkable findings, published in the journal Biology Open, shed light on cell differentiation and pave the way for future advancements in targeted tissue culturing for cell replacement therapies.
While most people make their career choices in their teenage years, stem cells make this life-altering decision just days after the fusion of egg and sperm. This is when gastrulation begins and the germ layers, known as the ectoderm, mesoderm, and endoderm, form. These layers eventually develop into our internal organs.
The fate of a stem cell in the early embryo is determined by a cocktail of signaling molecules, including BMP, FGF, Wnt, and Nodal. This cocktail, mixed by the surrounding extraembryonic tissue, determines whether the stem cell becomes a heart cell or a nerve cell, among other possibilities. While the composition of these signal cocktails is well understood, the role of fibroblast growth factor (FGF), which plays a crucial role in stem cell migration and growth, has remained largely unclear.
For the first time, Christian Schröter and his team have revealed that FGF acts as an antagonist to the signal molecule BMP. When FGF levels are low, BMP has a strong effect, leading to the development of heart cells and extraembryonic mesoderm. On the other hand, when FGF levels are high, the effect of BMP is suppressed, resulting in the growth of cells in the posterior body axis.
In the past, parents played a significant role in shaping their children’s career choices. Similarly, developmental biologists believed that stem cells were guided by external signals in a similar instructive process. However, the researchers’ findings suggest that stem cells have the ability to take control of their own fate. Even when instructed to become heart cells through continuous FGF addition in a culture dish, groups of completely different cell types still emerged. This suggests that stem cells communicate with each other, influencing their neighboring cells to develop in the same direction and become the same cell type.
“Our research contributes to a better understanding of the process of cell differentiation and the key players involved,” explains Christian Schröter. “In the future, these findings could potentially help generate specific cell types from stem cells, allowing us to replace dead tissue after a heart attack. However, selective culturing of specific cell types is still a challenge that we need to overcome.”
In the ever-evolving field of stem cell research, this study opens up new possibilities for targeted cell differentiation and brings us one step closer to harnessing the full potential of stem cells in regenerative medicine.