Plasmas, the powerful weapon against antibiotic-resistant pathogens, face a formidable opponent: bacteria armed with a heat shock protein. But fear not, a team of brilliant researchers led by Professor Julia Bandow and Dr. Tim Dirks from Ruhr University Bochum in Germany has made a groundbreaking discovery. They have uncovered the secrets of how bacteria overproducing the heat shock protein Hsp33 can withstand plasma treatment more effectively than their counterparts.
In their study, published in the esteemed Journal of the Royal Society Interface, the researchers revealed the specific components of plasma that activate the heat shock protein. When exposed to plasma, proteins lose their natural functions and clump together, rendering them toxic to cells. However, the mighty Hsp33, with its 33 kDa size, comes to the rescue by preventing this clumping and binding to unfolding proteins.
To test the protective powers of Hsp33, the researchers treated strains of bacteria that overproduce the protein with the Cinogy plasma source, commonly used in dermatology. The results were astonishing. These strains not only survived better than their wild-type counterparts after a short one-minute treatment but also remained active even after three minutes. However, prolonged exposure to plasma eventually led to the inactivation of the Hsp33-producing cells.
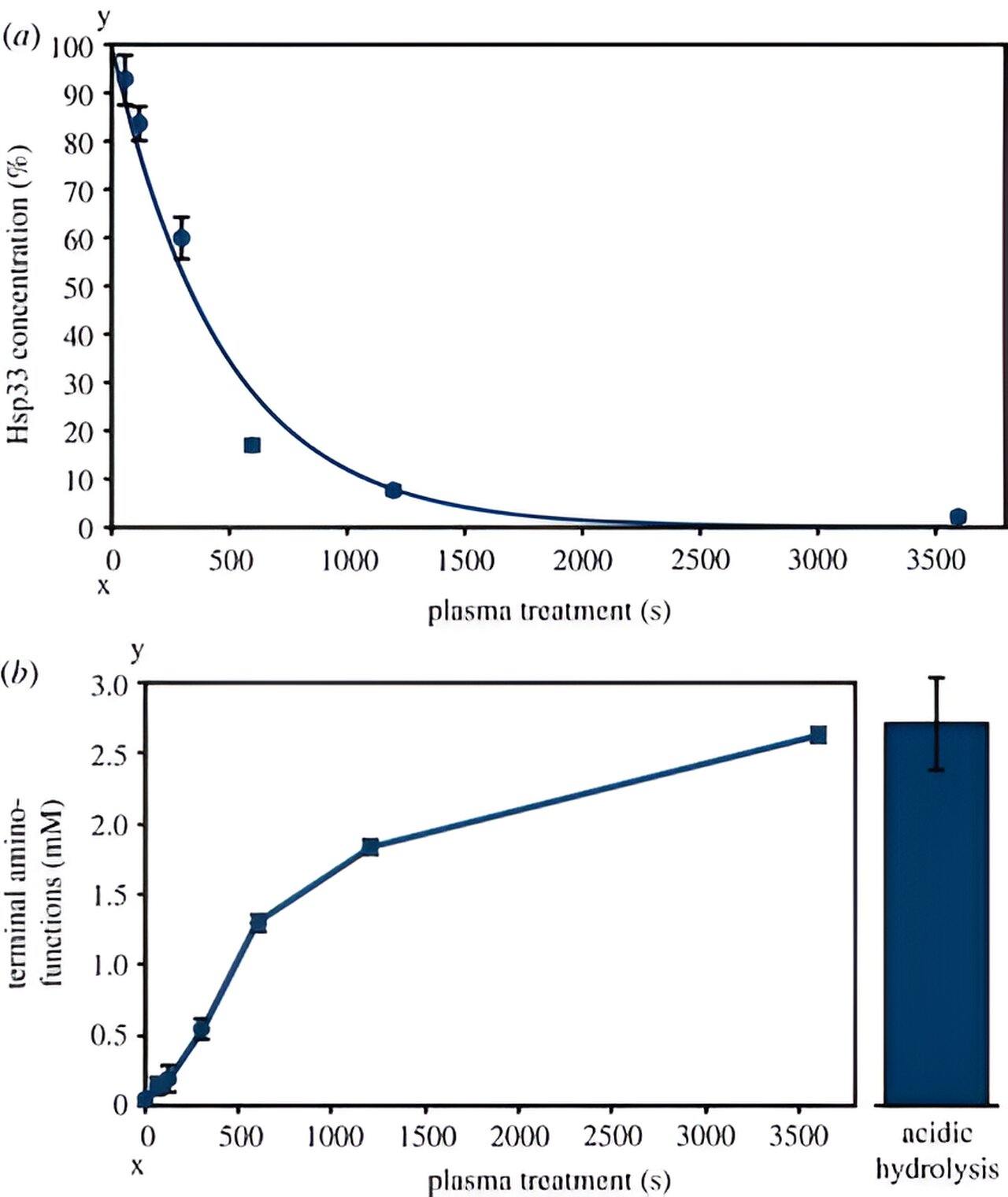
The team also investigated the activation of Hsp33 by subjecting the purified protein to the plasma source. They discovered that the protein’s activation is linked to oxidation and unfolding, which can be reversed. However, longer plasma treatment times of one hour resulted in the complete degradation of Hsp33. Additionally, the plasma negatively affected the protein’s ability to bind a zinc atom, which is crucial for maintaining its inactive state.
Curious about which plasma-produced species can activate Hsp33, the researchers conducted experiments using various stressors known to be produced by plasma. They found that Hsp33 responds to superoxide, singular oxygen, and atomic oxygen, but not to hydroxyl radicals and peroxynitrite. This valuable insight sheds light on the interaction between these species and bacterial cells. For instance, superoxide, one of the first species generated during oxidative stress, acts as a signaling molecule for bacteria, indicating further oxidative stress.
In conclusion, this groundbreaking research not only reveals the impressive defense mechanisms of bacteria against plasma treatment but also provides valuable knowledge about the activation of Hsp33. With this newfound understanding, scientists can develop more effective strategies to combat antibiotic-resistant pathogens and pave the way for innovative wound treatments.