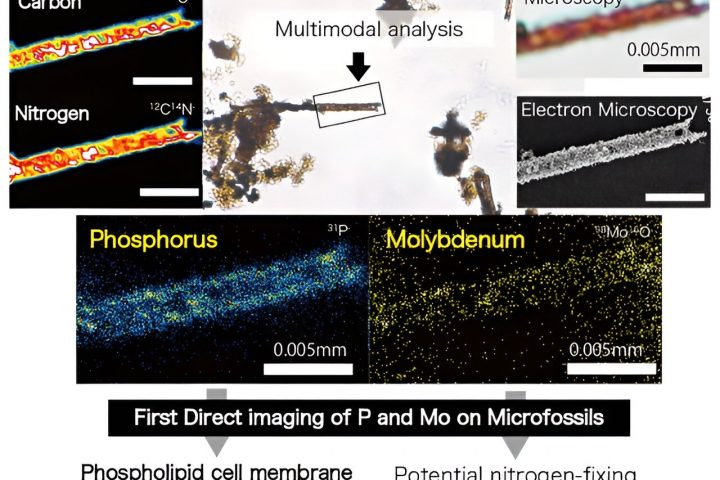
When it comes to color, size matters – especially at nanoscales. While at our level, objects of the same material have the same color regardless of size, the world of nanotechnology tells a different story. For years, scientists struggled to create nanostructures with predicted quantum effects. However, a breakthrough came from an unexpected source – the study of glass.
Glass, with its ever-changing colors seen in cathedral windows and precious objects, was traditionally made using the same compounds. It was through altering the manufacturing process that different colors were achieved. Dr. Alexey Ekimov, a recent Nobel Prize winner in Chemistry, sought to understand why the same compounds could produce varying hues. His research revealed that nanocrystals of different sizes were responsible for the diverse colors.
Another key figure in the field, Louis Brus, was experimenting with crystals of cadmium sulfide that could capture sunlight for chemical reactions. He discovered that crystals of different sizes reacted to light in distinct ways. This marked the beginning of quantum dots, a breakthrough that has revolutionized various applications.
Quantum dots act as a bridge between atoms and the materials we encounter in our daily lives. These tiny particles, made up of just a few thousand atoms, exhibit properties governed by quantum mechanics. If enlarged to the size of a basketball, a quantum dot would be as large as the Earth. Their minuscule size allows quantum mechanics to dominate, but with a different effect compared to single atoms or small molecules.
“Quantum dots are tiny particles of semiconductors that are so small that the properties of electrons in them are determined by the laws of quantum mechanics. Because of that, the properties of quantum dots are nothing like those of the atoms or the big crystals that eventually they grow into,” explained Professor Bawendi from MIT.
One remarkable property of quantum dots is their ability to absorb and re-emit light in different colors, depending on their size. This unique characteristic makes them valuable for applications such as LEDs, energy-harvesting, and light-harvesting technologies. They also hold promise in fields like chemistry, biology, and medicine. Researchers are even exploring the possibility of using quantum dots to track and potentially eliminate cancerous tissue in the body.
While the potential applications of quantum dots are vast, both Professor Bawendi and Professor Brus refrain from making specific predictions. As Professor Brus wisely stated, “It is extremely hard to predict the future of technology.”
To learn more about quantum dots and their implications, watch the full interview with Professor Mongui Bawendi in the video above or by following this link.