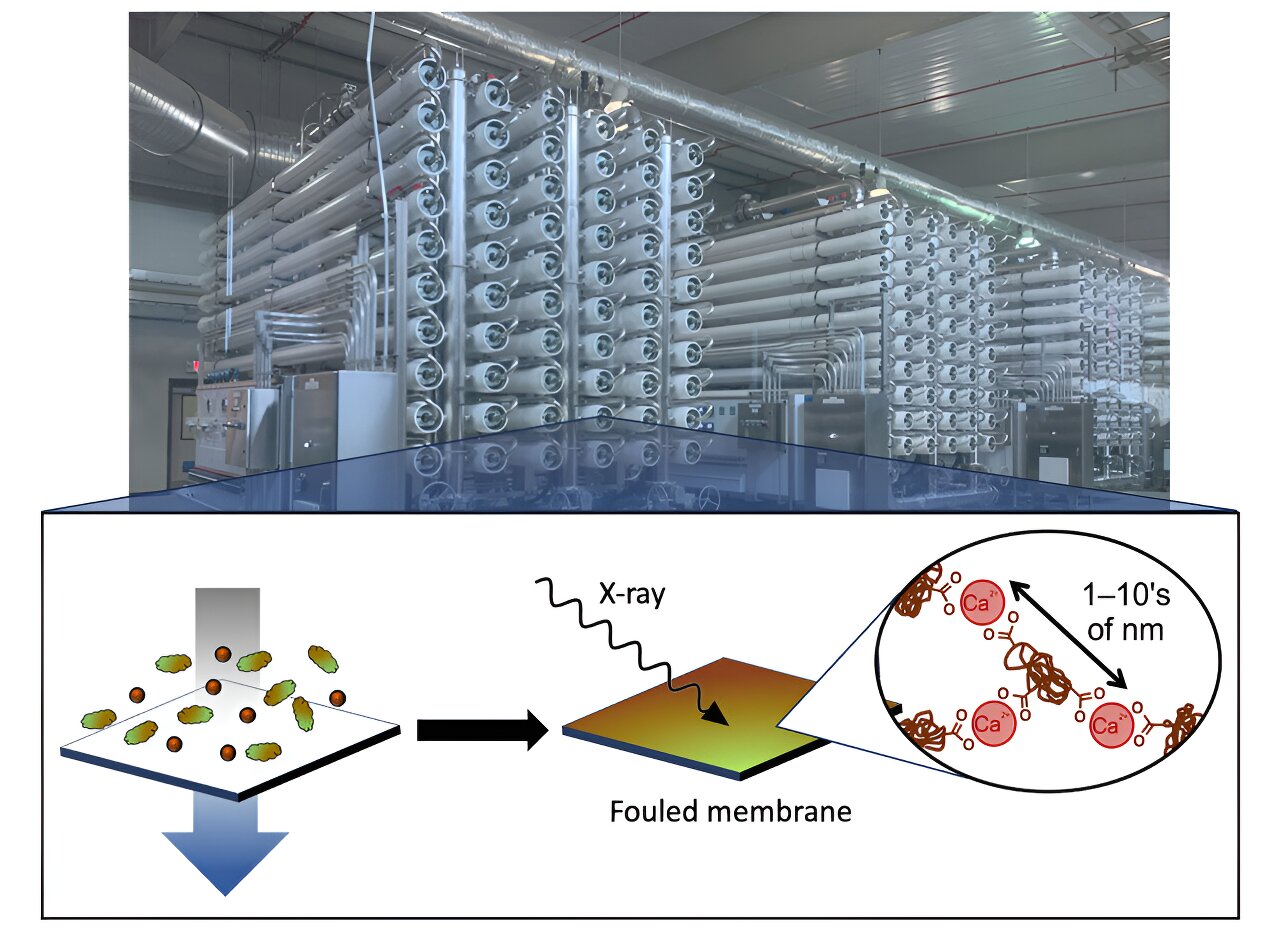
When it comes to water treatment, using a membrane can be a game-changer. However, there’s a catch. Over time, junk builds up on the membrane surface, making the treatment less efficient. This process is known as fouling. In a groundbreaking study recently published in the Journal of Membrane Science, researchers delved into the fascinating world of membrane fouling. They specifically focused on the interactions between natural organic matter and positively charged ions, like calcium cations, commonly found in water due to dissolved minerals and salts.
“Fouling has always been a major challenge in water treatment since the advent of membranes,” explained Matthew Landsman, the study’s first author and an ALS collaborative postdoctoral fellow from the University of Texas at Austin’s Center for Materials for Water and Energy Systems (M-WET), a DOE Energy Frontier Research Center (EFRC). “Our research aimed to unravel the molecular-level mechanisms behind membrane fouling caused by natural organic matter. By doing so, we can establish design rules to create better membranes.”
The team conducted laboratory fouling experiments on membranes at UT Austin. They then utilized synchrotron characterization techniques at the Advanced Light Source (ALS) and Brookhaven’s National Synchrotron Light Source II (NSLS-II) to analyze the composition of the fouled membranes. At ALS Beamline 7.3.3, wide angle X-ray scattering (WAXS) was employed to detect any inorganic contaminants, such as calcium carbonate, that may have precipitated on the membranes. At NSLS-II, soft and tender X-ray scattering experiments were conducted to determine the distribution of calcium in the fouling layers.
One of the mechanisms they studied involved calcium cations forming bridges between organic molecules, causing them to aggregate and form a thick fouling layer. This layer restricted the amount of clean water that could pass through the membrane. In “synergistic” fouling experiments, where mixtures of organic and inorganic foulants were used, the fouling was less severe than expected based on the individual effects of each contaminant. This suggests that interactions between contaminants in complex foulant mixtures can not only contribute to more fouling, but they can also compete with each other and result in less overall fouling.
“There’s a lot of fascinating water chemistry at play here,” Landsman remarked. “These findings have given us valuable insights into how we can tackle the treatment of complex water sources, which are becoming increasingly important in meeting today’s growing water needs.”
,,,