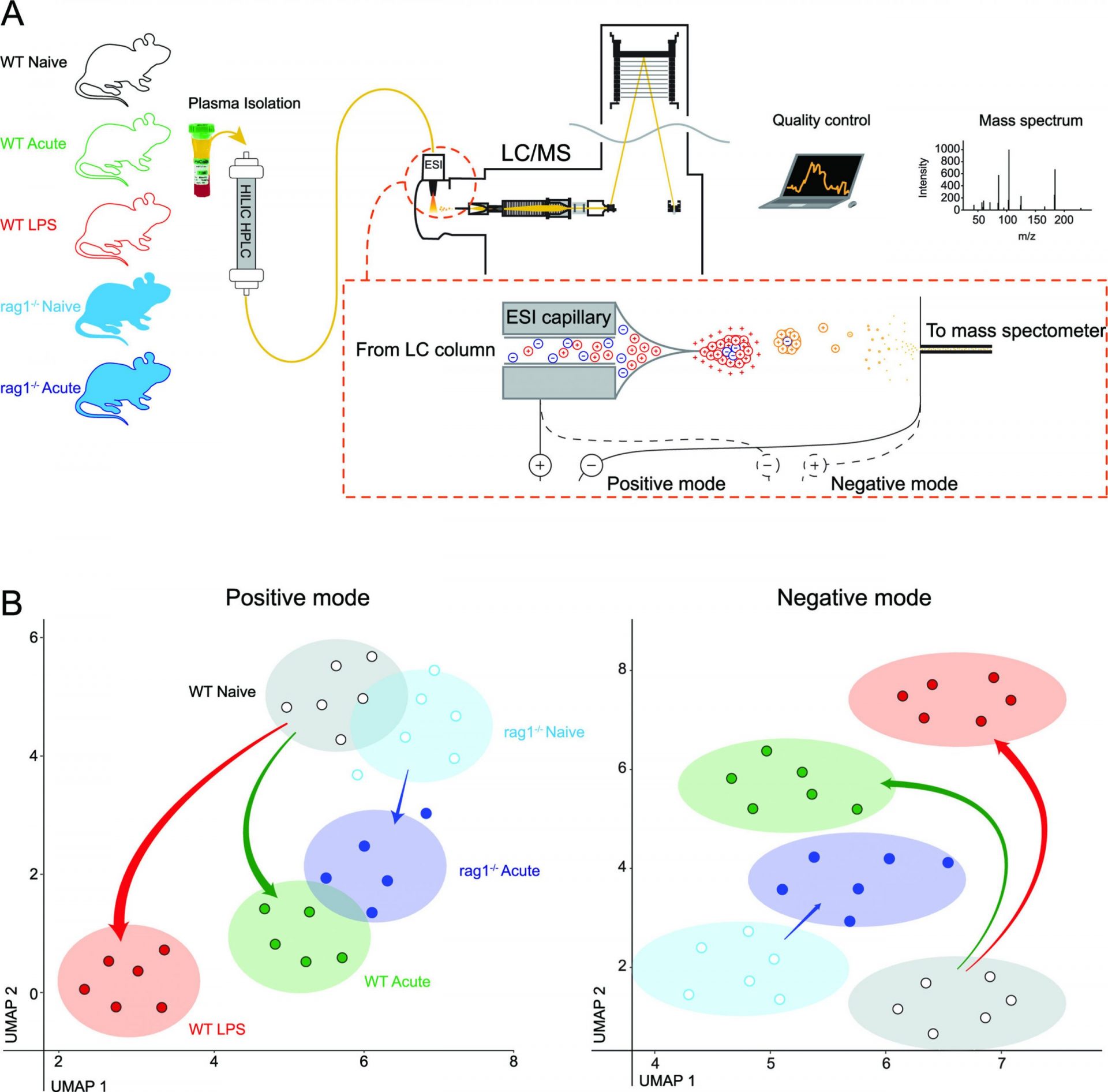
Exciting new research conducted by the Peter Doherty Institute for Infection and Immunity (Doherty Institute) and the Kirby Institute has uncovered a potential breakthrough in the fight against malaria. The study reveals that inflammation in the body can actually slow down the development of malaria parasites in the bloodstream, offering a promising strategy for preventing or limiting severe disease.
A mosquito-borne disease, malaria is caused by Plasmodium parasites that invade and multiply within red blood cells. Previous research has shown that these parasites can sense and respond to conditions within the host by aligning with their internal body clocks. While the impact of the body’s nutrient levels and circadian rhythms on parasite development is known, the influence of host inflammation has remained a mystery—until now.
This groundbreaking animal-model study, published in the journal mBio, reveals that when the body’s immune system responds to inflammation, it alters the chemical composition of the plasma, directly hindering the maturation of Plasmodium parasites as they circulate in the bloodstream.
Associate Professor Ashraful Haque, Laboratory Head and co-lead of the Bacterial and Parasitic Infections theme at the Doherty Institute, and one of the senior authors of the paper, emphasizes the captivating dynamic of the host-parasite relationship. “First, we discovered that inflammation in the body prevented the early stage of the parasites from maturing. We also noticed significant changes in the composition of the plasma due to inflammation—we were actually quite surprised by the magnitude of these changes,” said Associate Professor Haque.
Further investigation revealed substances in the altered plasma that are believed to inhibit parasite growth in the body. This discovery unveils a new mechanism that slows down the development of malaria parasites in the bloodstream. While the research was conducted using animal models, the team is eager to explore if similar inhibitory mechanisms occur in humans.
Dr. David Khoury, Lead of the Malaria Analytics Group at the Kirby Institute and co-senior author of the paper, highlights the remarkable response of the parasites to changes in their environment. “Parasites residing in red blood cells rapidly sense and respond to their new environment, showing fascinating adaptability. Using cutting-edge genome sequencing technology, we observed that even after just four hours in this changed plasma, the parasites adjusted their genetic and protein activity, resulting in slower maturation within red blood cells. It’s almost like the parasites actively sense an inhospitable host environment and trigger a coping mechanism,” said Dr. Khoury.
This study is the first to demonstrate that inflammation can alter the genetic behavior of individual parasites in the body. Professor Miles Davenport, Program Head of the Infection Analytics Program at the Kirby Institute and co-senior author of the paper, explains the potential benefits of this research on the interaction between systemic host inflammation and malaria parasite maturation. “This study, while based on animal models, broadens our understanding of malaria. It provides a foundation for further investigations into the specific mechanisms involved in the modulation of parasite maturation by inflammation and opens avenues for future studies to explore the identified inhibitory factors, genetic changes, and their implications for malaria development,” said Professor Davenport.
The ultimate goal of this work is to inform the development of new strategies to control, prevent, and reduce the burden of malaria, which affects over 240 million people globally. The research was conducted in collaboration with scientists from the Doherty Institute, The Kirby Institute, QIMR Berghofer Medical Research Institute, Wellcome Sanger Institute (UK), and Monash Institute of Pharmaceutical Sciences.