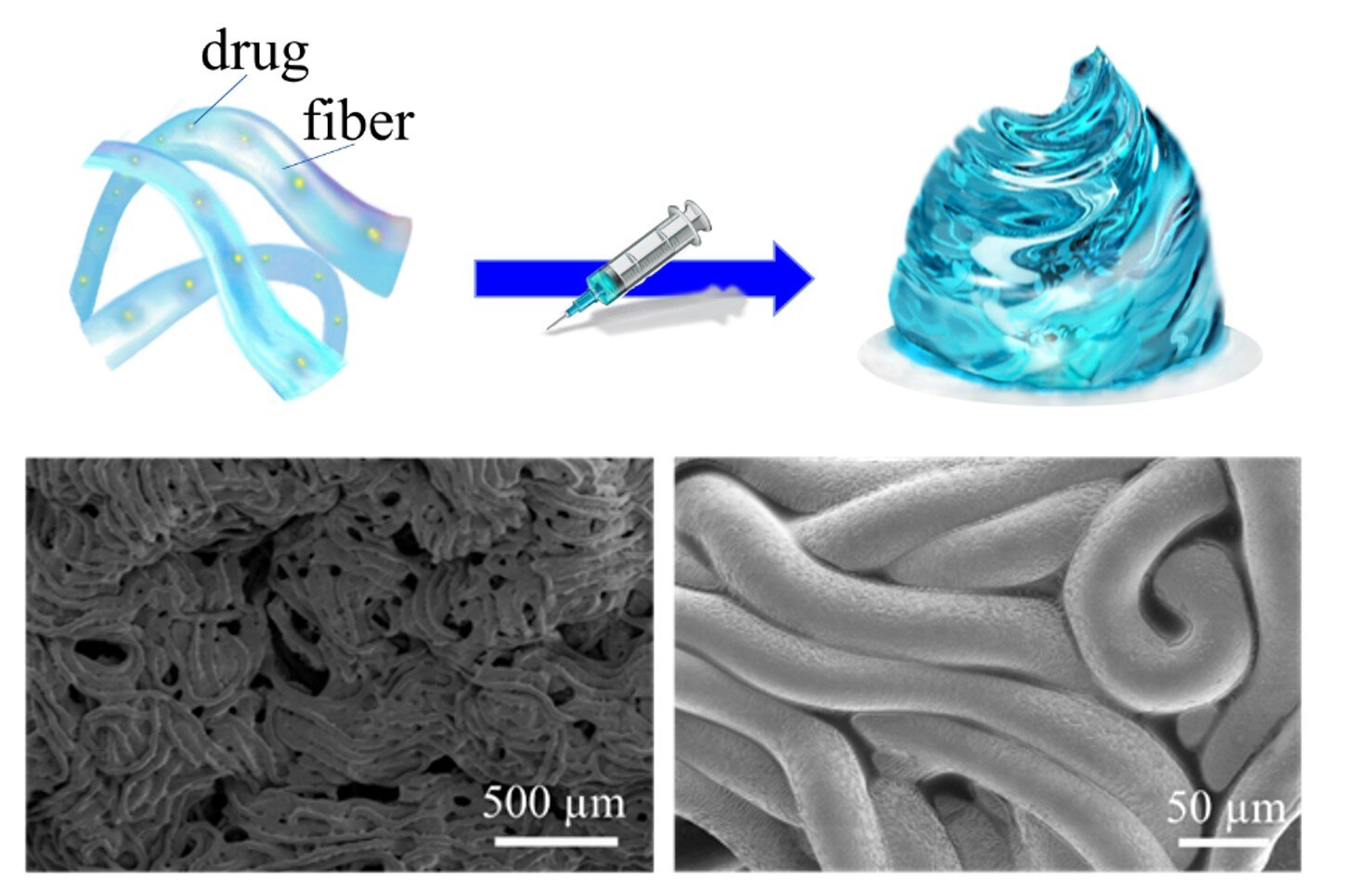
Prepare to be amazed by the groundbreaking innovation achieved by researchers from the Department of Mechanical Engineering at the University of Hong Kong (HKU). They have developed an injectable hydrogel called Fibro-Gel that overcomes the challenges of scalable manufacturing and on-demand drug release. But that’s not all – Fibro-Gel also demonstrates exceptional biocompatibility and the potential for vascularization.
The research team, led by Professor Anderson Ho Cheung Shum of the Department of Mechanical Engineering at HKU, collaborated with Professor Michael To’s team from The University of Hong Kong-Shenzhen Hospital, as well as the group of Professor Howard Stone and Dr. Janine Nunes from the Department of Mechanical and Aerospace Engineering at Princeton University.
This groundbreaking discovery has been published in Advanced Materials.
Injectable hydrogels are already valuable in wound healing due to their injectability, minimal invasiveness, and adaptability to irregular sites. However, the challenges of scalable manufacturing, matching properties, and on-demand drug release have limited control over biophysical cues for endogenous cell direction.
But fear not! The research team has designed and fabricated an oil-free, reaction-free, biomimetic, injectable, and fully scalable Fibro-Gel for wound healing applications. And here’s the exciting part – Ph.D. student Yanting Shen and postdoctoral fellow Dr. Yuan Liu discovered that Fibro-Gel’s physicochemical properties and drug release profiles can be precisely tailored by adjusting the microfiber lengths.
They found that the mechanical properties of Fibro-Gel vary depending on the length of the microfibers. Shorter microfibers result in lower stiffness and a more fluid-like behavior, leading to faster rates of drug release. On the other hand, longer microfibers exhibit higher stiffness and a more solid-like behavior, resulting in slower drug release rates.
With this controllable feature, the researchers have designed a drug release system that can release multiple drugs at different rates simultaneously, addressing the limitations of existing hydrogel systems. Extensive in vitro testing has shown the favorable biocompatibility of Fibro-Gel and its potential to aid vascularization.
This discovery is a game-changer in the field of drug delivery via hydrogel. Along with its controllable physicochemical properties that suit different tissues, Fibro-Gel holds tremendous potential for the development of advanced therapies and treatment modalities.
To put Fibro-Gel to the test, the researchers conducted in vivo tests using a mice excision skin model. The results are mind-blowing – Fibro-Gel promotes wound healing with an accelerated rate of new tissue regeneration and the appearance of de novo regenerated healthy tissue, outperforming a commercial gel. The researchers also demonstrated that the release of distinct drugs at different rates can further enhance wound healing using a two-layer Fibro-Gel model.
“Our research on Fibro-Gel has provided valuable insights into the controlled release rates of drugs and the tunable physicochemical properties essential for wound healing,” said Ph.D. student Yanting Shen. “This innovative approach has significantly accelerated the rate of healing, resulting in the formation of intact new tissue.”
Professor Shum is optimistic about the collaborative effort, stating, “Our multidisciplinary research collaboration has enabled us to address the topic of wound healing from multiple perspectives, leading to the development of Fibro-Gel. This novel hydrogel has immense potential to address critical medical needs that require more versatile and compatible soft materials.”
This successful partnership between HKU and Princeton University, supported by the Research Impact Fund by the Research Grants Council of Hong Kong, exemplifies the power of cross-border collaboration and highlights the significance of pooling expertise to drive groundbreaking research in the field of regenerative medicine.
Professor Shum and his team are dedicated to pushing the boundaries of scientific and engineering discovery and fostering innovation in the biomedical field. He believes this research opens up new possibilities for material systems with extensive biomedical applications, including therapeutic agent delivery for infectious diseases and tissue regeneration.