Hydrogen gas is gaining momentum as the fuel for a clean and green future. This carbon-neutral fuel source releases massive amounts of energy through combustion, producing water vapor as a by-product. One popular method of hydrogen production is the splitting of water using electricity.
An electrochemical cell is used to split water, releasing hydrogen gas through the hydrogen evolution reaction (HER). Catalysts are employed to enhance the efficiency of this process by reducing the HER overpotential.
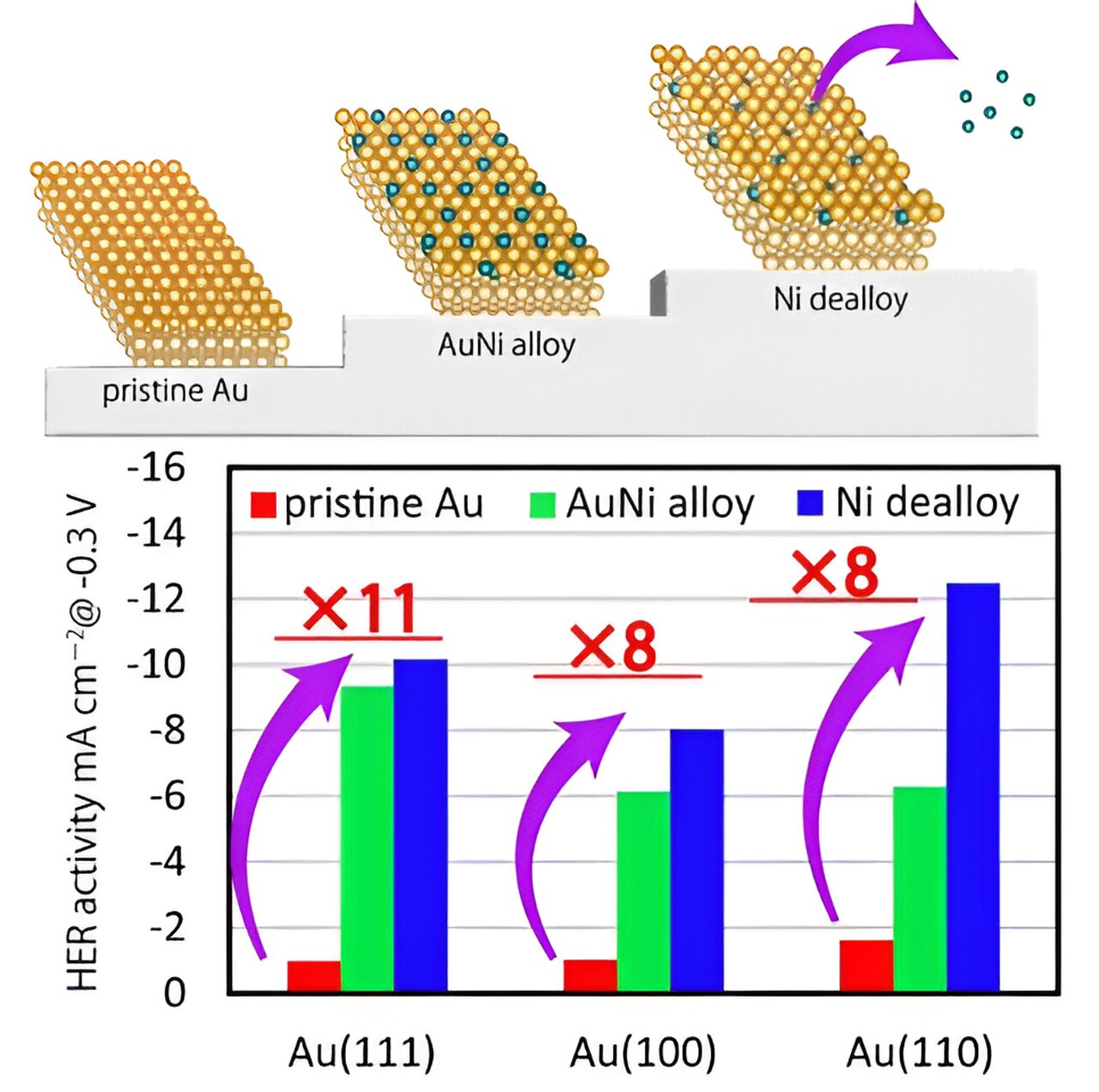
A recent study has shown that an alloy of gold (Au) and nickel (Ni) exhibits promising HER activity. While the electrochemical properties of AuNi have been extensively studied, little is known about its surface structure and atomic composition, which play a crucial role in determining its electrocatalytic activity.
A team of researchers from Chiba University has made significant progress in understanding AuNi electrocatalysts. In their groundbreaking article published in ChemElectroChem, the team investigated the surface structure, atomic arrangement, and HER activity of AuNi surface alloys prepared at different temperatures.
Dr. Nakamura, the lead researcher, explains the motivation behind the study. “Expensive metals like platinum are commonly used as catalysts for water electrolysis. However, AuNi nanoparticles have emerged as a promising non-platinum alternative. It is essential to further improve their HER activity.”
The team conducted various measurements and analyses to evaluate the HER activity and surface properties of the AuNi/Au catalyst. They found that the HER activity depended on the surface structure of the Au substrate, with the (110) surface exhibiting the highest activity.
The surface alloy also enhanced the HER activity through a process called Ni dealloying. This was confirmed by XPS and SXRD analyses, which revealed a decrease in the atomic occupancy on the topmost layer of the surface. The defects created by the Ni dealloying process activated the HER.
This study provides valuable insights into the structural and electrochemical properties of AuNi surface alloys, paving the way for highly active and durable Au-based catalysts for practical applications in electrolysis and fuel cells. “Designing effective non-platinum electrocatalysts can reduce the cost of water electrolysis and improve its energy conversion efficiency, which is crucial for transitioning to a hydrogen-driven society,” concludes Dr. Nakamura.