The production of hydrogen could become more efficient with the addition of a carefully chosen salt. Researchers at the Leiden Institute of Chemistry (LIC) and the University of Twente have discovered that the type of salt used in the electrolysis process affects the formation of hydrogen bubbles. This is significant because bubble formation directly impacts the efficiency of hydrogen production.
Sustainable hydrogen production is set to play a crucial role in the energy transition. Hydrogen is a CO2-free energy carrier and a raw material for various applications, such as fertilizers. “Electrolysis is a sustainable method for producing hydrogen,” says Marc Koper, professor of Catalysis and Surface Chemistry.
What is electrolysis?
In electrolysis, water (H2O) is converted into hydrogen (H2) gas and oxygen (O2) gas through an electrochemical reaction. To achieve this, two electrodes are placed in water containing dissolved salt, which enhances electrical conductivity. When an electrical voltage is applied to the electrodes, hydrogen bubbles form at one electrode and oxygen bubbles form at the other.
Why are small bubbles desirable?
Koper and his team investigated the formation and detachment of hydrogen bubbles from the electrode. Their research, published in the journal Nature Chemistry, revealed that it is preferable for small bubbles to form and detach quickly from the electrode surface. “Bubbles act as insulators, reducing the conductivity of the electrolysis cell and leading to less efficient hydrogen production,” explains Koper.
A carpet of small bubbles
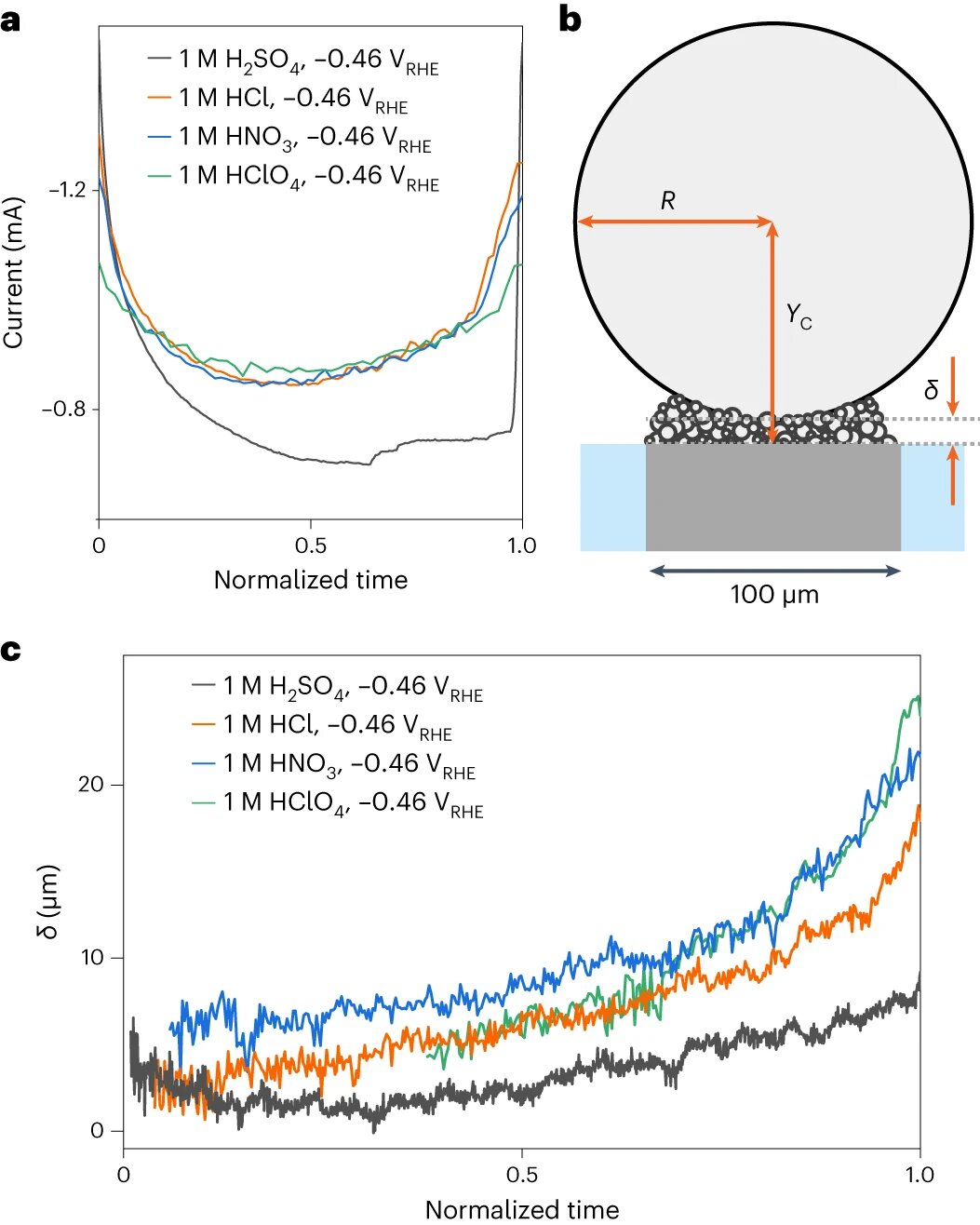
For their study, the scientists used a tiny electrode measuring about 0.1 millimeters, allowing them to observe the behavior of a single bubble with different types of salt. “This enabled us to study how different salts affect bubble formation,” says postdoc Sunghak Park.
The Twente research group, led by Detlef Lohse and Dominik Krug, used advanced cameras to carefully record the behavior of the bubbles. They discovered that instead of a single bubble, a carpet of smaller bubbles forms, with a larger bubble on top being fed by the smaller ones.
The influence of salt on bubble formation
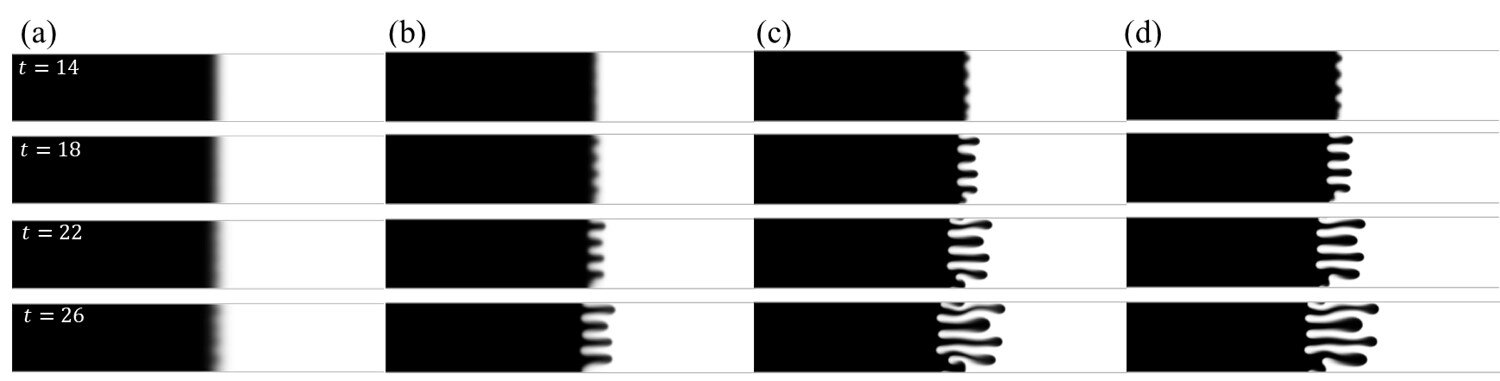
However, the most surprising finding was the significant influence of the type of salt used on bubble formation. “This had not been observed before,” says Koper. Salt consists of positively charged cations and negatively charged anions, and it was found that the anion specifically affects bubble formation. With sulfate anion, small bubbles easily merge into a larger bubble, while with perchlorate anion, mainly small bubbles form and quickly detach from the surface.
What does wine tasting have to do with electrolysis?
The researchers discovered that this phenomenon is due to the Marangoni effect, which is also responsible for the formation of wine legs or “tears.” The Marangoni effect creates a fluid flow caused by a concentration or density difference. In electrolysis, the production of hydrogen creates a difference in anion concentration at the electrode, resulting in a similar fluid flow.
The direction of this flow depends on the type of anion. With sulfate, the flow pushes the bubbles downwards, against the electrode, causing the larger bubble to grow and remain in place longer. With perchlorate, the flow pushes the bubbles away from the electrode, preventing them from merging into a larger bubble. This leads to a faster release of small bubbles from the surface.
Enhancing the efficiency of commercial electrolysis
“The role of this mechanism in bubble formation was previously unknown,” says Koper. “It’s fascinating because it means we can control bubble behavior by using the right salts.” Further research will determine whether this discovery can improve the efficiency of commercial electrolysis.