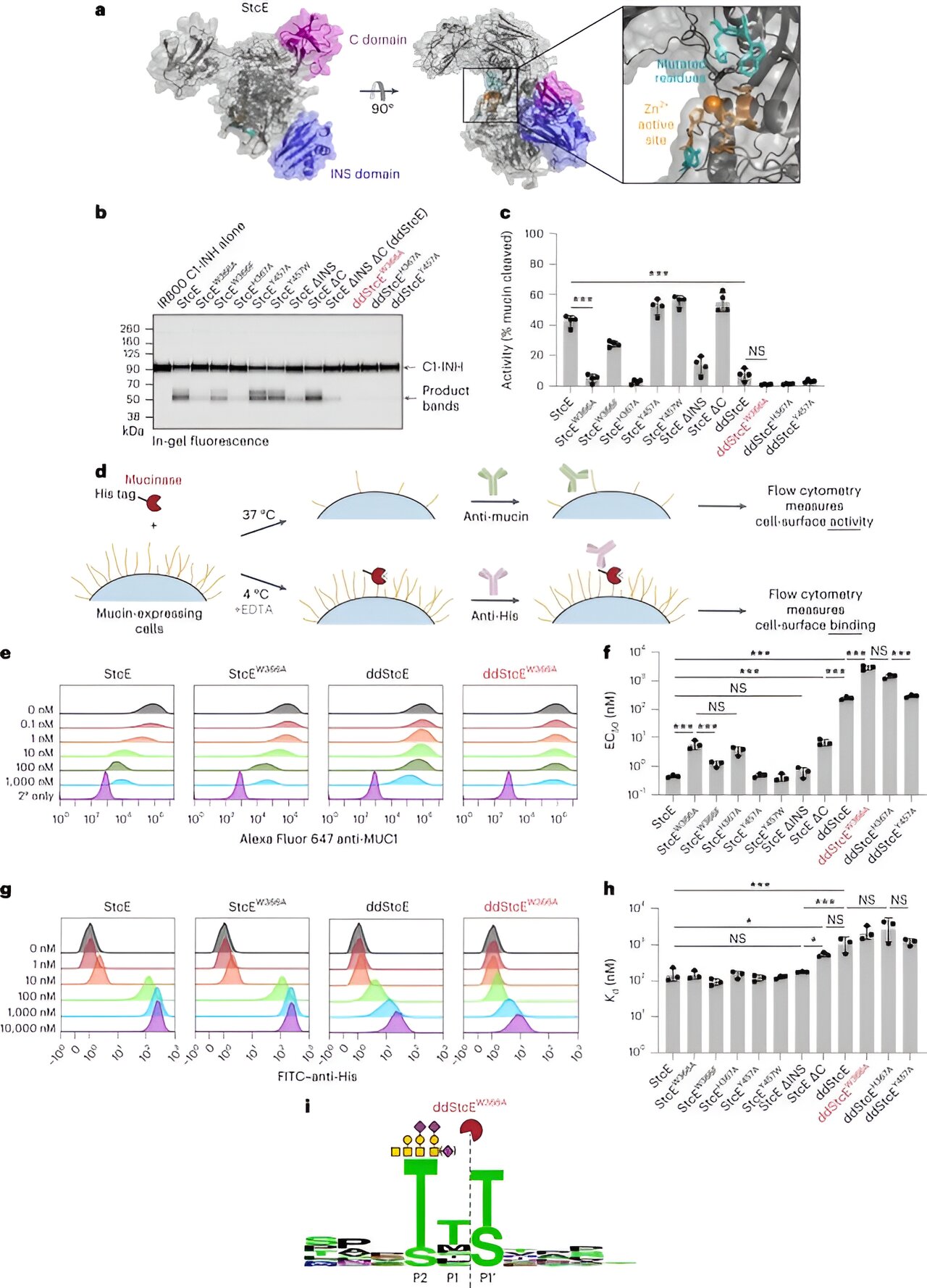
Cancer cells have a sneaky way of evading the body’s immune defenses by taking advantage of a group of molecules called mucins. But now, researchers at Stanford have made a groundbreaking discovery: they’ve engineered a biomolecule that specifically removes mucins from cancer cells. This finding could have a significant impact on future cancer therapies.
Mucins are proteins coated with sugar that normally help defend the body against physical insults and pathogens. However, cancer cells can hijack mucins to aid their own survival. While cutting off mucins from cancer cells seems like a potential therapy, it’s challenging because mucins exist in various forms on all mammalian cells. Indiscriminately targeting mucins could have unintended consequences.
But the Stanford-led research team has come up with a solution. They’ve created a biomolecule that acts like a pair of scissors, composed of a mucin-cutting enzyme called a mucinase fused to a cancer-cell-targeting nanobody. This two-part biomolecule selectively prunes mucins associated with specific cancer cells, leaving healthy cells unharmed.
In lab-grown human cancer cells and mouse studies simulating breast and lung cancer, the researchers found that the biomolecule treatment significantly reduced tumor growth and increased survival. Their findings, published in Nature Biotechnology, have wide-ranging implications as mucins are linked to various diseases, including cystic fibrosis, respiratory diseases, and viruses.
“We discovered that by targeting this mucinase to cancer cells and using it to remove mucins, we achieved a therapeutic benefit,” said senior author Carolyn Bertozzi, a professor at Stanford’s School of Humanities and Sciences.
The study’s co-lead author, graduate scholar Gabrielle “Gabby” Tender, worked alongside two former researchers from Bertozzi’s lab—Kayvon Pedram, a group leader at HHMI’s Janelia Research Campus, and D. Judy Shon, a postdoctoral scholar at Caltech.
When good mucins go bad
While mucins are generally beneficial, cancer cells exploit them for their own gain. Tender explains, “Cancers take advantage of mucins, which play important roles in our bodies, such as forming mucus in our gut and lungs, to protect themselves and spread throughout the body.”
This study focused on two functions of mucins that promote cancer progression. The first function helps cancer cells survive in low-adhesion environments, allowing them to break off and spread to other parts of the body. Most cells cannot survive in such environments, but mucin-modified cells can.
The second function involves mucins binding to checkpoint receptors, which are like immune system guard dogs that inspect cells. Some cancer cells decorate their surfaces with mucins coated in specific sugars that bind well to these receptors. When this sugar-decorated mucin binds to checkpoint receptors, it tricks the immune system into ignoring the cancer cells instead of destroying them.
Getting there is half the battle
The researchers’ mucin-seeking biomolecule consists of two fused parts. The first part is a mucinase derived from bacteria that cuts mucins. The second part is a cancer-specific nanobody that binds to an antigen on cancer cells.
The nanobody acts as a guide, directing the mucinase to the cancer cell. This technology is part of a larger program at Sarafan ChEM-H that focuses on proximity-based medicines, which bring biomolecules together for targeted chemical reactions.
The team extensively studied bacterial proteases that cleave mucins, eventually selecting a mucinase called StcE derived from E. coli. They engineered an “engineered sticky” version of StcE, called eStcE, that specifically targets tumor-associated mucins.
For the nanobody, they chose one known as 5F7, which corresponds to the HER2 antigen associated with breast, ovarian, and other cancers. The researchers designed different orientations of the eStcE mucinase-HER2 nanobody combo and tested each for effectiveness.
In lab tests and studies using mice, the researchers found that the biomolecule selectively killed target cancer cells and significantly reduced tumor growth, leading to increased survival rates.
Future directions
While this study is a major step forward in cancer research, there is still work to be done before it can be developed into a therapy for humans. The researchers plan to create a comparable targeted mucinase using a protease derived from humans to minimize the risk of unwanted immune responses.
Despite the need for further research, this discovery offers hope in the fight against cancer. “We finally have an approach to degrade mucins on cancer cells,” said Tender. “This opens up new possibilities for treatment.”