Carbon Capture, Utilization, and Storage (CCUS) technology holds immense potential for reducing CO2 emissions and creating renewable energy storage solutions. One crucial aspect of this technology is the CO2 reduction reaction (CO2RR), which can convert CO2 into carbon-based fuels and chemicals.
Recently, a team of researchers from the University of Science and Technology of China (USTC) made a groundbreaking discovery about the mechanism of CO2RR on nickel (Ni) single-atomic sites. Their study, titled “Asymmetric Dinitrogen-Coordinated Nickel Single-Atomic Sites for Efficient CO2 Electroreduction,” was published in Nature Communications.
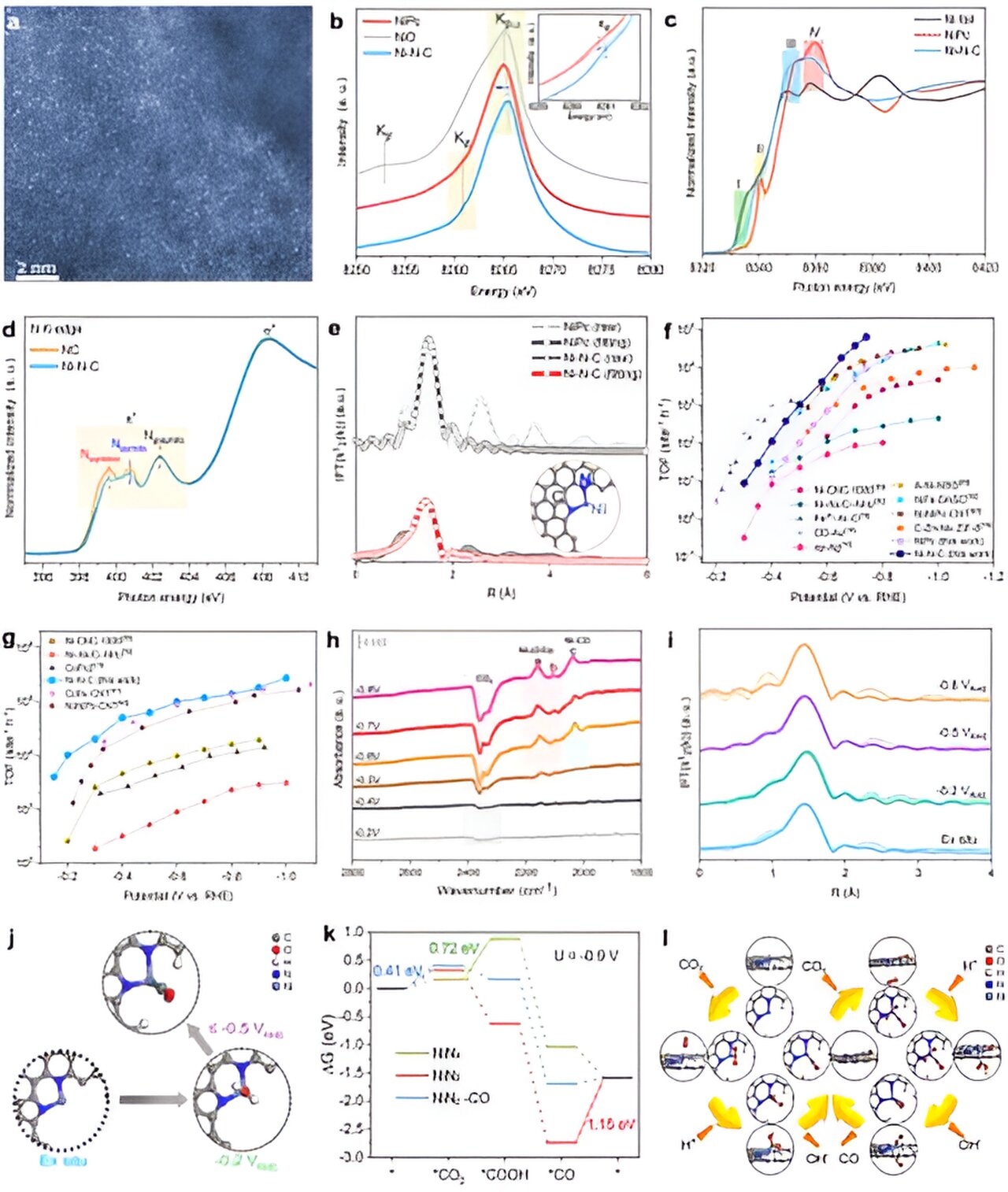
Traditional catalysts for CO2RR, such as gold (Au) and silver (Ag), have limitations like high cost and low current density. This hinders the efficiency of the reaction. To overcome these challenges, the researchers developed an asymmetric dinitrogen-coordinated nickel single-atom catalyst (Ni-N-C). This catalyst demonstrated exceptional performance in CO2 electroreduction reactions in both neutral and alkaline conditions.
The study revealed that the Ni-N-C catalyst achieved a CO partial current density of 20.1 mA cmgeo-2 at -0.15 V vs. reversible hydrogen electrode (VRHE). It also exhibited a Faraday efficiency of over 90% for CO in the potential range of -0.15 to -0.9 VRHE and a high turnover frequency (TOF) of over 274,000 site-1 h-1 at -1.0 VRHE. These results surpassed most reported catalysts.
This study provides valuable insights into the role of catalysts in CO2 electroreduction and paves the way for future advancements in CO2 reduction technologies.