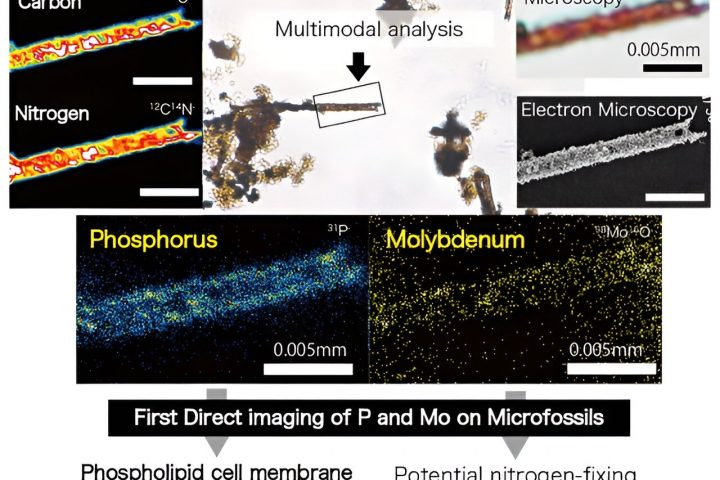
Did you know that iron doesn’t just rust when it comes into contact with oxygen and water? Some bacteria have the ability to decompose iron anaerobically through a process called electrobiocorrosion.
The bacterium Geobacter sulfurreducens, which resides in sediment, utilizes electrically conductive protein threads to decompose iron. In a study published in the journal Angewandte Chemie, researchers discovered that Geobacter produces magnetite from the iron, which further promotes corrosion in a positive feedback loop.
Bacterial biofilms are responsible for microbial metal corrosion, causing more damage than any other biofilm-related issue. Electrobiocorrosion is often caused by bacteria found in river sediments, such as the anaerobic genus Geobacter.
Geobacter doesn’t rely on atmospheric oxygen for respiration. Instead, it draws energy from the transfer of electrons from iron, resulting in the formation of magnetite. Until now, the exact mechanism of Geobacter’s iron corrosion has remained a mystery.
A team of researchers from Northeastern University in Shenyang, China, led by Dake Xu, conducted a detailed investigation into the mechanism of electrobiocorrosion. They hypothesized that electrically conductive pili, thin filaments that grow out of the bacteria, play a crucial role in this process. Geobacter forms “e-pili” from conductive proteins, which act as electric wires, conducting electricity. However, it was unclear whether the e-pili could directly withdraw electrons from metal surfaces.
To test their hypothesis, the researchers allowed two strains of Geobacter to grow on a stainless-steel surface until biofilms formed. One strain formed conductive e-pili, while the other strain produced pili made from less conductive proteins due to genetic modification.
The results showed that the strain with e-pili performed significantly better on the steel plate. It grew more and created deeper pits in the metal, indicating higher consumption. The team also observed a corrosion current, a direct indication of iron oxidation.
The researchers concluded that the bacteria with e-pili formed an “electrical connection” to the metal. Even bacteria located further away in the biofilm, not in direct contact with the metal, were able to obtain electrons using e-pili.
Additionally, the team investigated the influence of magnetite, a mineral formed during the corrosion of iron, on microbial corrosion. They found that adding magnetite to the biofilm not only increased the growth of Geobacter but also resulted in a stronger corrosion current on the metal surface.
“The finding that magnetite, a common corrosion product, facilitates electrobiocorrosion has significant corrosion implications,” the team emphasized. They recommend considering the propensity of materials to form magnetite when attempting to improve corrosion protection in the future.