Exciting Breakthrough: New Microscopy Method Reveals How Molecular Aggregates Form in Living Cells
In a groundbreaking study, researchers from Freiburg and Cambridge have successfully quantified the formation of small, dynamic molecular aggregates in living cells for the first time. These aggregates play a crucial role in signal processing, making this discovery a significant advancement in cell biology.
Many vital processes in cells occur within membraneless molecular aggregates, which ensure that the necessary molecules are present in the right concentration and proximity. Until now, observing and analyzing the formation of these condensates in living cells has been challenging. However, scientists from the Cluster of Excellence CIBSS at the University of Freiburg and the University of Cambridge have developed a new microscopy method that allows them to study these condensates in real-time.
Published in the prestigious journal Nature Communications, the researchers reveal that the formation of these condensates is not solely governed by physical forces but also by active biological mechanisms. To facilitate further research, they have made their experimental protocols and analysis tools freely available, enabling scientists in less advanced laboratories to study small aggregates.
Cells rely on the organization of molecules to function properly. Without specialized compartments, biochemical processes would be chaotic. While some compartments are separated by membranes, many others are membraneless. These membraneless molecular aggregates, known as condensates, are highly flexible in size and number, and they are believed to form through a process called “liquid-liquid phase separation.”
“Condensates are crucial control mechanisms in cells as they can regulate the speed of biochemical processes,” explains Prof. Thorsten Hugel, a member of the Cluster of Excellence CIBSS. He led the study alongside Prof. Aleks Reinhardt from the University of Cambridge. While large and static condensates have been extensively studied, the researchers focused on small, dynamic condensates that are more biologically intriguing.
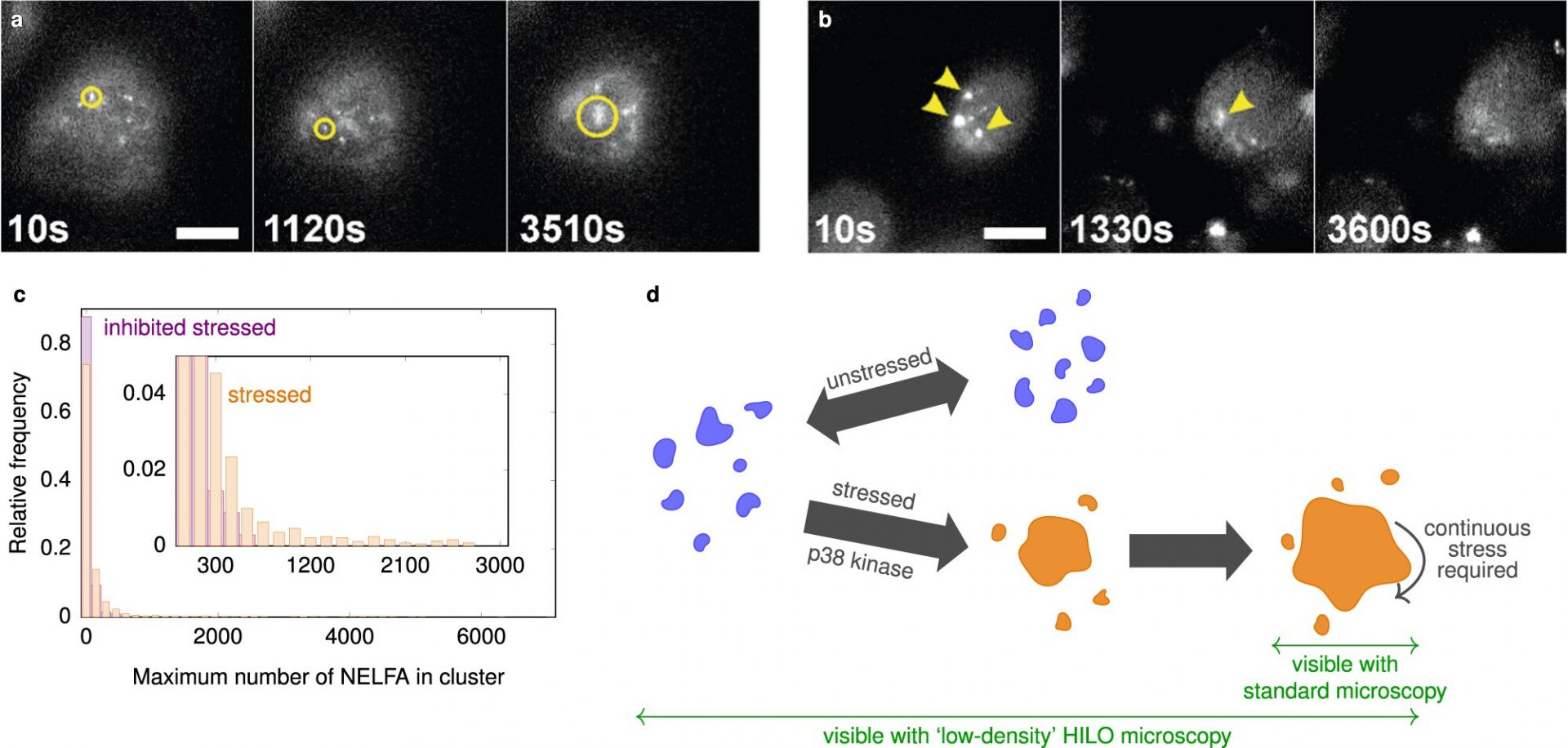
Studying small condensates has been challenging due to their size and rapid dynamics. However, the researchers overcame these technical limitations by combining high-resolution fluorescence microscopy with a tilted laser, known as HILO microscopy, along with a special experimental procedure and AI-based analysis methods.
Their findings deviated from conventional theoretical descriptions of condensate formation. While the initial growth of the condensates followed expected physical models, their growth suddenly stopped once they reached a certain size. The researchers also investigated the protein NELF and discovered that its condensates play a role in stress response and gene expression inhibition.
Interestingly, the researchers observed that small NELF condensates exist even in non-stressed cells. This suggests that the cell actively keeps these condensates small until stress signals trigger rapid growth. Understanding these mechanisms is crucial for efficient stress responses, especially as we age and face age-related neurodegenerative diseases.
Protein aggregates, like NELF condensates, are believed to have fundamental functions in signal processing within cells. The newly developed microscopy method opens up avenues for comprehensive research into these functions and their implications in misfolding diseases such as dementia and neurodegenerative diseases like Alzheimer’s and Huntington’s.
Ultimately, a deeper understanding of these mechanisms could lead to improved disease diagnosis and the development of effective therapies.