The demand for images of nanoscale structures inside living cells is on the rise, as they provide valuable insights into cellular structure and function. Until now, the tools for capturing such images have been limited. However, a team of researchers led by Takeshi Fukuma and Takehiko Ichikawa at Kanazawa University have developed a groundbreaking protocol for using atomic force microscopy (AFM) to image inside living cells. Their findings have been published in the journal STAR Protocols.
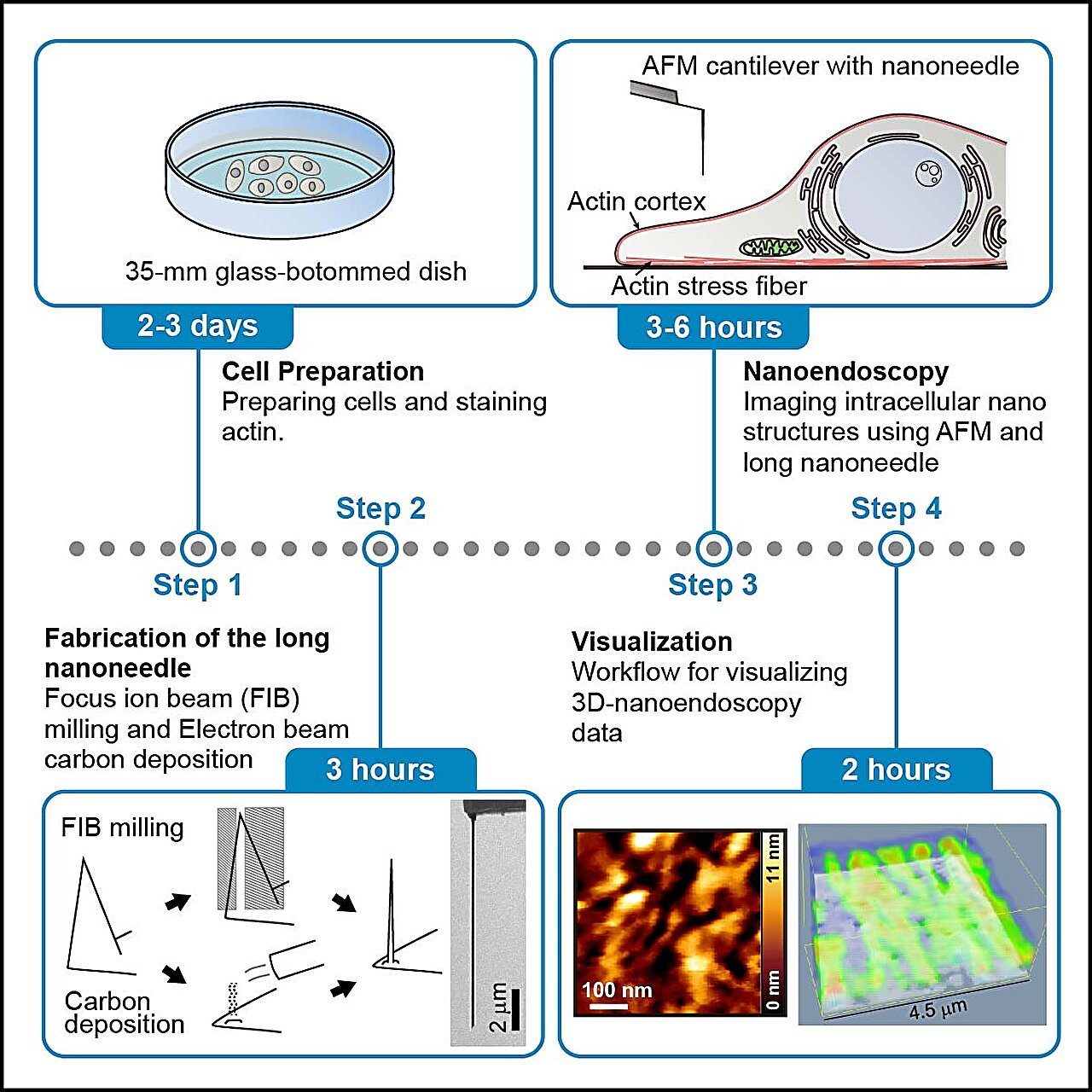
AFM, which was first developed in the 1980s, utilizes changes in forces between a sample surface and a nanoscale tip to produce high-resolution images of surface topography. Over time, the technique has become more advanced, allowing researchers to extract information about samples at speeds that enable the capture of moving images at the nanoscale. However, AFM has been limited to surface imaging. Other techniques, such as electron microscopy, can provide a view of the inside of a cell, but they are not compatible with living cells due to their operating conditions. Fluorescence microscopy, on the other hand, is commonly used on living cells, but it faces practical challenges when it comes to imaging at the nanoscale. AFM, however, overcomes these limitations and, by incorporating a nanoneedle to penetrate cells, Fukuma, Ichikawa, and their team have successfully demonstrated its ability to image inside cells at the nanoscale, which they refer to as nanoendoscopy-AFM.
In their protocol, the researchers outline four stages for nanoendoscopy-AFM. The initial steps involve preparing the cells, staining them with a fluorescent dye, and checking the fluorescence to quickly identify the imaging area. The next stage involves fabricating the nanoneedles, which can be done through either etching with a focused ion beam or building them up with electron beam deposition. Following this is the nanoendoscopy stage itself, with the researchers providing details on both 2D and 3D nanoendoscopy approaches. The protocol also includes instructions for cleaning up after capturing the nanoendoscopy images, as well as data processing techniques for visualizing the measured data. Each stage is accompanied by helpful tips for successful execution and troubleshooting guidance for when things don’t go as planned.
This technique is expected to be suitable for observing intact intracellular structures, including mitochondria, focal adhesions, endoplasmic reticulum, lysosomes, Golgi apparatus, organelle connections, and liquid-liquid phase-separated structures. The researchers conclude that this protocol has the potential to become a standard tool for studying nanoscale structures.