Researchers at the University of Wisconsin–Madison stumbled upon a fascinating discovery while studying a strain of yeast closely related to the kind used in beer fermentation. They were amazed to find that the yeast left behind half of its genetic material as it evolved.
Their groundbreaking study, recently published in the journal PLOS Biology, initially aimed to investigate a strain of wild yeast called Saccharomyces eubayanus. This wild yeast is a parent to the hybrid yeast strains used in lager-style beer fermentation. One crucial characteristic of yeasts used in beer production is their ability to break down maltose, the primary sugar in beer brewing materials.
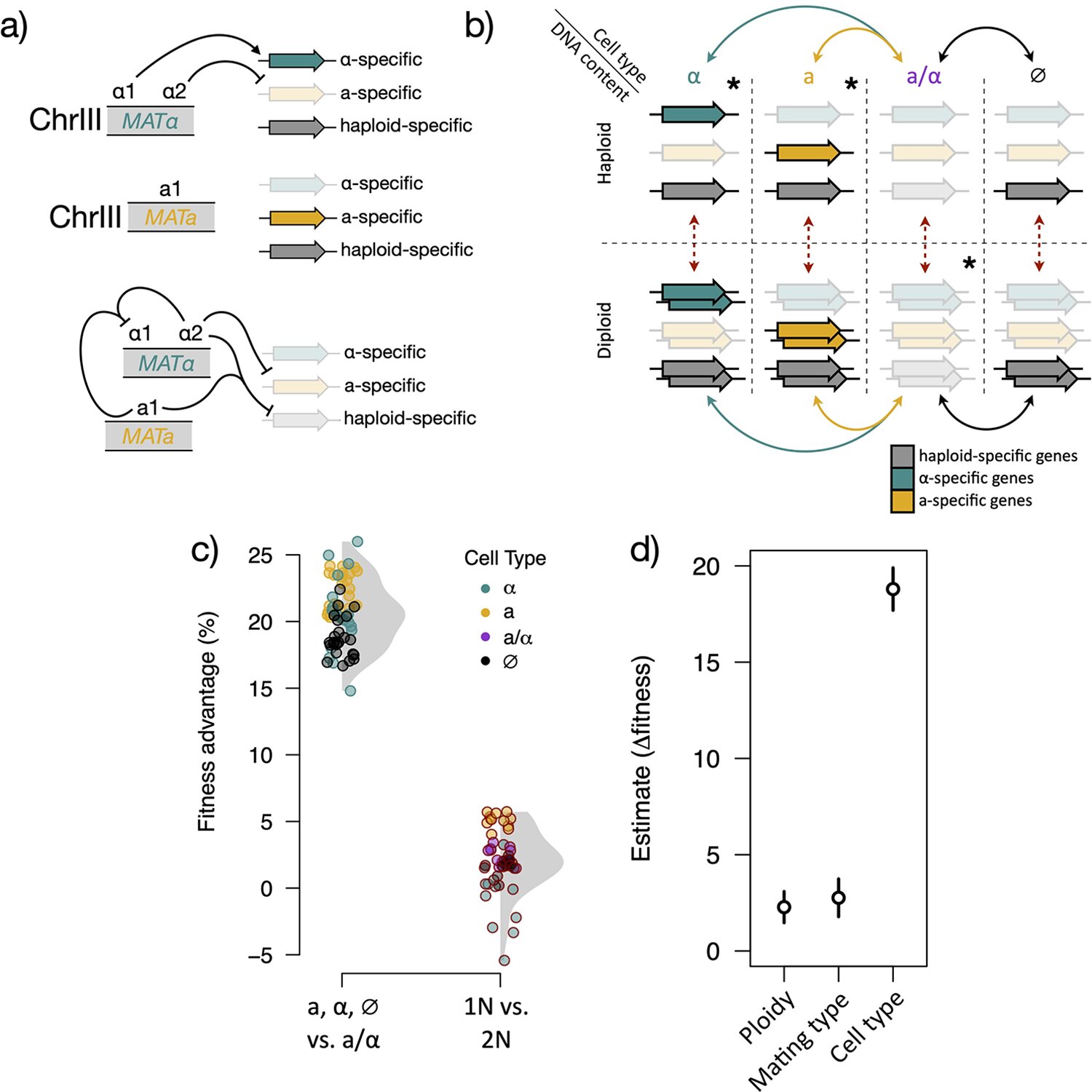
The researchers selected a yeast strain that had lost the ability to metabolize maltose despite possessing the necessary genes. Through evolution experiments, they grew the yeast on maltose and selected the descendants that thrived. Surprisingly, the S. eubayanus yeast regained the ability to consume maltose. However, the exact changes in the yeast remained a mystery.
“We were puzzled because we couldn’t pinpoint the specific genetic changes in these evolved isolates that had improved their maltose metabolism,” says John Crandall, the first author of the paper and a doctoral student in the lab of Chris Todd Hittinger, a genetics professor at UW–Madison. It turned out that the change was far more dramatic than a simple mutation in a single gene. The yeast had actually lost half of its genetic material during the experiment.
Specifically, all the yeast’s chromosomes lost their pairs, transitioning from diploids (with two copies of each chromosome) to haploids (with a single copy of each chromosome). The number of chromosome sets, known as ploidy, is a fundamental aspect of organismal biology. “It defines the structure and content of the genome, which then has all sorts of implications for life cycle and evolutionary trajectories,” explains Crandall.
While ploidy changes in yeasts have been observed before, they typically involve haploids becoming diploids, not the other way around. Moreover, ploidy changes in yeasts often lead to changes in cell type, which directly impact sexual reproduction. However, in this case, the ploidy change affected sugar metabolism instead.
“We had to disentangle various factors, such as absolute ploidy, cell type, and their contributions to fitness [growth and reproduction]. This intrigued us because there are different theoretical frameworks explaining how absolute ploidy can impact fitness,” says Crandall. “But there wasn’t a good model for why cell type should affect fitness in this condition, which is why it’s particularly surprising that it did.”
This new study provides valuable insights into the mechanisms behind ploidy changes that directly influence growth and metabolism. These findings have the potential to revolutionize various fields, including medicine, biofuel production, and industries involving fungi.
“In many pathogenic, clinically relevant, and industrially important species, cell type or ploidy could represent an unexplored dimension of trait variation,” says Crandall.