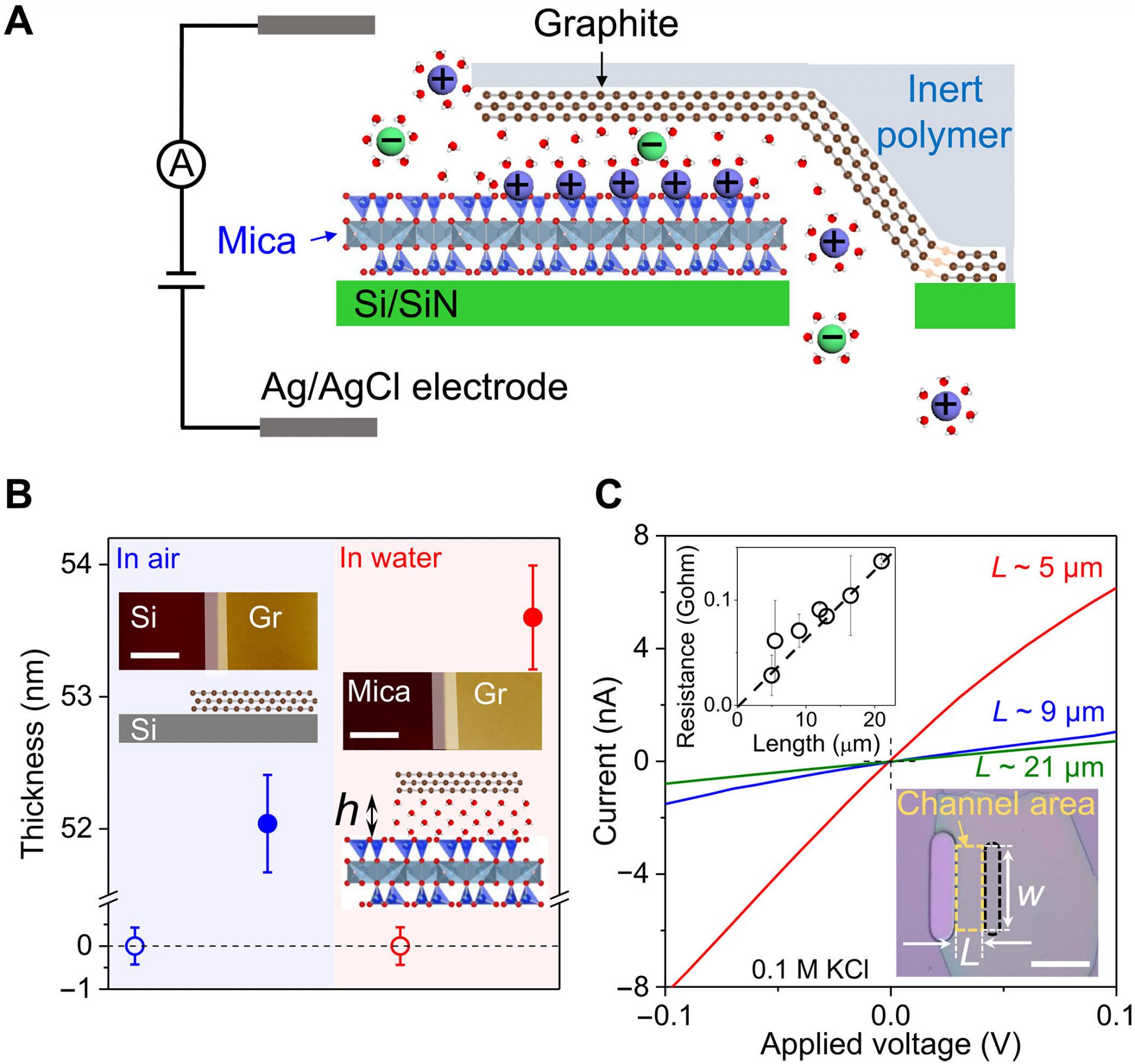
Materials scientists have been fascinated by the potential of nanofluidic channels in filtration technologies and osmotic energy harvesting. These channels have the ability to allow fast ion permeation, but the underlying mechanisms are still not fully understood. However, a recent report published in Science Advances by Yu Jiang and his research team in China sheds light on this topic.
Jiang and his team developed two-dimensional nanochannels with walls made of atomically flat graphite and mica crystals. These unique wall structures allowed them to investigate the interactions between ions and the interior surfaces. They discovered that ion transport within these channels was significantly faster than in bulk solutions, providing valuable insights into the effects of surface properties on ion transport at the nanoscale.
The mechanisms of nanoscale ion transport can outperform their macroscale counterparts in terms of transport rates. For example, ion flow through protein channels in cell membranes is crucial for the functioning of life. Nanoporous membranes are also used for water purification, ion separation, and osmotic power generation. To understand the mechanisms of fast ion transport at the nanoscale, researchers need to create nanochannels with well-regulated geometry and interior structures.
Jiang and his team focused on investigating the origin of fast ionic transport within nanochannels that contained ion adsorption sites in the interiors. They designed the channels in a way that minimized the chance of contamination, allowing them to study the effects of adsorption on pristine surfaces. They assembled graphite and mica crystals and transferred them to silicon substrates, aligning the layers with the aperture to create the nanochannels.
The scientists used an atomic force microscope to measure the thickness of the graphite and mica surfaces in the channel region. They also filled the reservoirs with chloride solutions of different concentrations to create a concentration gradient. Through their experiments, they were able to measure the ionic conductivity of potassium chloride and investigate the surface effects on ion transport.
The results showed that the high conductance and selective ion adsorption on mica surfaces indicated significant surface diffusion. The scientists developed a quantitative expression for ion transport in the graphite-mica channels, focusing on the transport of monovalent cations. They found that surface diffusion played a crucial role in providing high ionic conductivity in nanofluidics.
This research opens up new possibilities for creating nanochannels using mica group crystals, which have preferences for adsorbing diverse cations. These channels can be used for ion transport and sensing applications, as they can distinguish ions based on their adsorption energies.
In conclusion, Yu Jiang and his colleagues have made significant advancements in understanding fast ion transport in nanofluidic channels. Their research provides valuable insights into the surface effects on ion transport at the nanoscale and opens up new possibilities for applications in filtration technologies and energy harvesting.